Article Text
Abstract
Objective To evaluate the in vitro antimicrobial effects of cyclodextrin-complexed and uncomplexed Manuka honey on bacteria commonly associated with blepharitis, and in vivo rabbit eye tolerability of a cyclodextrin-complexed methylglyoxal (MGO) Manuka Honey microemulsion (MHME).
Methods and analysis In vitro phase: Bacterial growth inhibition was assessed by area under the growth curve (AUC) for Staphylococcus aureus, and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for S. aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa with cyclodextrin-complexed and uncomplexed Manuka honey were determined.
In vivo phase: Six rabbits were administered 20 µL of MHME (at 1:10 dilution) to the right eye (treated) and 20 µL of saline to the left eye (control) daily, for 5 days. Tear evaporation, production, osmolarity, lipid layer, conjunctival hyperaemia and fluorescein staining were assessed daily, before and 15 min after instillation.
Results In vitro phase: The relative AUC for cyclodextrin-complexed Manuka honey was lower than that of uncomplexed honey at both 250 and 550 mg/kg of MGO (both p <0.05). Cyclodextrin-complexed honey had lower MIC and MBC than uncomplexed honey for both S. aureus and S. epidermidis, but not P. aeruginosa.
In vivo phase: No significant changes were observed in the parameters assessed in either treated or control eyes (all p >0.05).
Conclusion Overall, antimicrobial potency of cyclodextrin-complexed Manuka honey was greater than uncomplexed honey. No significant immediate or cumulative adverse effects were observed with MHME application on rabbit eyes, supporting future conduct of clinical safety and tolerability trials in human subjects.
- Blepharitis
- Manuka honey
- cyclodextrin
- methylglyoxal
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Blepharitis is a common chronic condition characterised by eyelid inflammation. Although the pathophysiology remains uncertain, bacterial overcolonisation is implicated with ocular inflammatory responses. Topical antibiotics and corticosteroids are currently prescribed to treat inflammatory exacerbations; however, concerns regarding drug resistance and adverse effects necessitate development of alternative therapeutic options. New Zealand Manuka honey exhibits antimicrobial and anti-inflammatory properties that suggest potential for the treatment of blepharitis. Complexation with α-cyclodextrin has previously been reported to enhance the antibacterial effects of Manuka honey.
What are the new findings?
In clinically applicable concentrations, cyclodextrin-complexed MGO Manuka Honey (Manuka Health New Zealand) generally exhibited greater in vitro antimicrobial effects than uncomplexed honey on bacteria commonly associated with blepharitis. In vivo safety and tolerability evaluation on rabbit eyes demonstrated that a cyclodextrin-complexed Manuka honey microemulsion (MHME), designed for topical application, did not result in significant immediate or cumulative adverse effects.
How might these results change the focus of research or clinical practice?
The results of this study support progress to clinical safety and tolerability trials of MHME eye cream in human subjects.
Introduction
Blepharitis is a common chronic condition that predisposes the eyelids to bacterial overcolonisation and inflammation, causing significant morbidity worldwide.1 2 Clinically, the condition can be differentiated into anterior blepharitis, which affects the anterior lamella of the eyelid and the eyelashes; or posterior blepharitis, most commonly meibomian gland dysfunction, which affects the posterior lamella.3 The pathophysiological mechanisms of blepharitis remain uncertain. However, overcolonisation of the eyelid region, with bacteria such as Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa,4–7 has been implicated in the development and exacerbation of ocular surface inflammation.8 9 Increased bacterial lipase activity may promote tear film instability, leading to excessive tear evaporation and dry eye symptoms.8 10
While topical antibiotics may reduce bacterial load,11 their long-term use raises concerns with regard to drug resistance.12 Similarly, prolonged use of topical corticosteroids to manage ocular surface inflammatory episodes is contraindicated on account of the risk of side effects.1 9 As a result, the management of blepharitis remains limited largely to palliative therapies such as warm compresses and eyelid hygiene techniques.11 13 14 However, these therapeutic strategies are often perceived by patients as laborious and ineffective, and consequently are met with poor compliance.15 This highlights the need for a therapeutic intervention that attempts to address the bacterial and inflammatory aspects of the condition and is suitable for long-term application.
The inhibitory effects on ocular microbiota and anti-inflammatory properties of honey have previously been demonstrated, and are attributed to its high osmolarity, low pH, hydrogen peroxide content and nonperoxide components, including methylglyoxal (MGO).16 New Zealand Mānuka honey (Leptospermum scoparium), in particular, shows potential as a novel therapy for the management of blepharitis, due to its high concentrations of MGO, which is more resistant to physiological inactivation by heat and catalases than antimicrobial peroxide components.17 Cyclodextrins are commonly used additives in topical formulations, which increase the stability and solubility of hydrophobic drugs in water.18 19 Manuka honey has been complexed with α-cyclodextrin to form the product MGO Manuka Honey, which has previously been reported to have greater antibacterial effects than uncomplexed honey.20
This preclinical study sought to investigate the in vitro effects of cyclodextrin-complexed and uncomplexed MGO Manuka Honey on the growth of bacteria commonly associated with blepharitis, including S. aureus, S. epidermidis and P. aeruginosa. The in vitro human corneal epithelial cell viability and in vivo rabbit eye tolerability of an MGO Manuka Honey microemulsion (MHME) designed for topical periocular application were also evaluated.
Materials and Methods
In vitro phase
Materials
Cyclodextrin-complexed and uncomplexed MGO Manuka Honey containing 250, 400 and 550 mg/kg MGO (Manuka Health NZ, Auckland, New Zealand) were evaluated in the in vitro phase. Cyclodextrin-complexed MGO Manuka Honey comprises α-cyclodextrin and Manuka honey solids, while uncomplexed Manuka honey comprises honey solids and water. Cyclodextrin-complexed and uncomplexed Manuka honey, and cyclodextrin, were resuspended in Difco BHI broth (Fort Richard Laboratories, Auckland, New Zealand) containing 0.1% w/v catalase (Sigma Aldrich, New South Wales, Australia) and incubated with shaking at 37°C for up to 60 min. Catalase neutralises hydrogen peroxide so that any effect on bacterial growth observed can be attributed to MGO.21
Bacterial strains
The effect of cyclodextrin-complexed and uncomplexed Manuka honey, and cyclodextrin on bacterial growth was tested against bacteria commonly associated with blepharitis, including S. epidermidis ATCC (American Type Culture Collection) 14990, S. aureus ATCC 6538 and P. aeruginosa ATCC 27853 (ATCC, Virginia, USA).
Time course of the effect on growth of S. aureus
A 17.8% w/v solution of catalase-treated cyclodextrin-complexed Manuka honey and a 9.7% w/v solution of uncomplexed Manuka honey containing honey with 250, 400 and 550 mg/kg MGO, and a 9.7% w/v solution of catalase-treated cyclodextrin were prepared. Both 17.8% and 9.7% w/v solutions of cyclodextrin-complexed Manuka honey and uncomplexed Manuka honey respectively contain 8% w/v honey solids, and a 9.7% w/v solution of cyclodextrin is equivalent to that present in a 17.8% w/v solution of cyclodextrin-complexed Manuka honey. Difco BHI broth alone was used as a control. Solutions were mixed with an equal volume of bacterial culture containing S. aureus ATCC 6538 at approximately 106 CFU/mL in Difco BHI broth, giving a total volume of 200 µL. Cultures were incubated in sterile, flat-bottomed, 96-well microplates (Sarstedt, Germany) for 24 hours at 37°C, with agitation at 100 rpm. Growth was measured as absorbance of the culture at 595 nm using a µQuant microplate spectrophotometer (BioTek, USA).
Minimum inhibitory and bactericidal concentrations for S. aureus, P. aeruginosa and S. epidermidis
Solutions of catalase-treated cyclodextrin-complexed Manuka honey and uncomplexed Manuka honey containing 200 mg/kg MGO were prepared from cyclodextrin-complexed Manuka honey and uncomplexed Manuka honey containing honey with 400 mg/kg MGO. Each was diluted with BHI broth to give solutions containing 25, 50, 75, 100, 125, 150 and 175 mg/kg MGO. Solutions of 4.7%, 9.5%, 14%, 19%, 24%, 28%, 33% and 38% w/v catalase-treated cyclodextrin were also prepared; these concentrations of cyclodextrin are equivalent to those present in MGO Manuka Honey containing 25, 50, 75, 100, 125, 150, 175 and 200 mg/kg MGO, respectively. BHI broth served as a control. At each concentration, 180 µL honey solution was mixed with 20 µL bacteria to give 107 CFU/well. Cultures of S. aureus ATCC 6538, P. aeruginosa ATCC 27317 and S. epidermidis ATCC 14990 were incubated in clear, flat bottomed, 96-well plates (Greiner Bio-One, Austria) for 16 hours at 37°C with agitation at 100 rpm. Growth was measured as absorbance of the culture at 600 nm using a μQuant spectrophotometer (Bio-Tech Instruments, USA). Growth inhibition was defined as an A600 nm below 0.05 after 16 hours of growth (equal to uninoculated broth control). Samples (10 µL) of minimum inhibitory concentration (MIC) cultures not exhibiting growth were inoculated onto BHI agar (Fort Richard Laboratories) and incubated for 16 hours at 37°C with 5% CO2 in air. Each dilution was tested against each bacterial species in triplicate on three separate occasions.
Formulation preparation and cell viability evaluation
An MGO MHME was developed and subsequently manufactured by Manuka Health New Zealand for in vivo evaluation. The microemulsion was prepared by mixing glycerol and polysorbate 80 (PS 80) before adding caprylic/capric triglyceride MI12 and phosphate buffered saline (PBS). Cyclodextrin-complexed MGO Manuka Honey 400 mg/kg (Manuka Health New Zealand) was then added to achieve a final MGO concentration of 100 mg/kg (MHME). For sterilisation, formulations were gamma irradiated (IR4; Atomic Energy, Canada) and osmolarity (VAPRO Vapor Pressure Osmometer; ELITech, New Zealand) and pH (Mettler Toledo pH Meter, New Zealand) of the formulations were determined before and after gamma exposure.
For the purpose of toxicity testing, human corneal epithelial cells (HCEC) were maintained in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 1 g/L glucose, 100 U/mL penicillin and 100 µg/mL streptomycin. HCEC were derived from human cadaver tissue obtained with consent, for research use, from the donor's family, and with approval from the Northern X National Ethics Committee (NTX/07/08/080/AM04). Cells were cultured at 37°C in 5% CO2-95% atmospheric air until confluent and seeded at a density of 1×104 cells/well. After 24 hours of attachment, 100 µL of diluted (in MEM) formulation excipients and MHME were added for 15 min. Cells were then washed and incubated with 100 µL of MTT solution at 37°C for 3 hours before adding 100 µL of acidified isopropanol and measuring the absorbance at 570 nm. Negative controls were cultured in medium only and cell viability was expressed as a percentage of the negative control.
In vivo phase (rabbit)
The in vivo phase was approved by the institutional animal ethics committee (UAAEC 001156).
Paired-eye tolerability evaluation
The 5-day prospective in vivo paired-eye tolerability evaluation was conducted on six healthy male New Zealand white rabbits (weighing 2.5±0.5 kg). The rabbits were treated with simultaneous daily administration of 20 µL of diluted MHME (1:10 with saline) to the right eye, and 20 µL of saline control to the left eye, for 5 consecutive days. The solutions were instilled into the lower fornix of the conjunctival sac using a positive displacement pipette. The eyelids were manually blinked several times following each instillation to facilitate solution distribution across the ocular surface.
Tear film and ocular surface evaluations were conducted at baseline, and 15 min following each instillation. The tests were conducted in ascending order of invasiveness to minimise the impact on tear film physiology for subsequent tests. Tear film lipid layer grade was assessed using the handheld Tearscope (Keeler, Berkshire, UK). Tear film lipid layer grading was based on the Guillon-Keeler grading system: grade 1, open meshwork; grade 2, closed meshwork; grade 3, wave/flow; grade 4, amorphous; grade 5, coloured fringes; grade 0, non-continuous layer (absent or abnormal coloured fringes).22 23
Tear evaporation rate was measured using an Evaporimeter EP3 (ServoMed, Sweden) within a modified goggle housing. Tear film osmolarity was evaluated with a clinical osmometer (TearLab, California, USA), using 50 nL tear samples collected from the lower lid tear meniscus. Aqueous tear production was evaluated using a phenol red thread hooked over the inferior lids into the fornices of the conjunctival sacs, with the wetted length measured after 15 s.
Conjunctival hyperaemia, corneal clarity and iris appearance were examined under a Burton lamp, and the severity graded clinically from 0 (none), through 1 (mild), 2 (moderate) to 3 (severe) judged to the nearest 0.25 increment. Sodium fluorescein dye was applied to the bulbar conjunctiva in order to evaluate the localised corneal and conjunctival areas of epithelial desiccation. Conjunctival and corneal staining was graded, respectively, on a 0–5 scale with increasing confluence in each area.24
Tear film osmolarity profile evaluation
On day 1 of the 5-day in vivo tolerability evaluation, osmolarity measurements of both eyes were taken at baseline, and at 1 min intervals following instillation of either diluted MHME or saline control, for 10 min.
Undiluted formulation safety evaluation
The MHME formulation was developed for topical application onto the periocular skin of closed eyelids, without direct contact to the ocular surface. However, to evaluate the effect of accidental application to the ocular surface, 20 µL of undiluted MHME formulation was instilled into the right eye of each rabbit, and 20 µL of saline control to the left eye simultaneously, following the conclusion of the 5-day in vivo tolerability evaluation. Ocular surface characteristics were assessed at baseline, and 30 s, 5 min and 10 min following instillation.
Statistics
Statistical analyses were performed using IBM SPSS Statistics V.22 and GraphPad Prism V.6.02. For the in vitro phase, area under the growth curve (AUC) values were calculated in GraphPad Prism V.6.02. AUCs were generated for each microplate well by plotting increasing culture absorbance (a measure of growth) against time. Comparisons of AUC values (as a relative quantity of control AUC) were conducted using Kruskal-Wallis tests, and post hoc multiplicity-adjusted Dunn's tests.
For the in vivo phase, repeated measures (RM) two-way analysis of variance (ANOVA) testing was performed to test the significance of treatment, time and interaction (treatment-by-time) effects on continuous measurements, and ordinal data were converted to rank values prior to undergoing analysis. Post hoc multiplicity-adjusted Sidak's tests were conducted to examine immediate treatment effects at each time point, and cumulative treatment effects over the treatment period. All tests were two tailed and p <0.05 was considered significant.
Results
In vitro phase
Microbiological evaluations
Summary statistics of the in vitro microbiology evaluation are presented in table 1. In the assessment of time course effects on S. aureus growth, cyclodextrin-complexed Manuka honey displayed effects at least as strong as uncomplexed honey at each concentration of MGO tested. Cyclodextrin-complexed honey effected a greater reduction in AUC at 250 and 550 mg/kg MGO (both p<0.01), but not 400 mg/kg MGO (p=0.98). Although cyclodextrin alone also reduced AUC, the effect was significantly weaker than cyclodextrin-complexed or uncomplexed honey at all concentrations of MGO tested (all p<0.01). The cyclodextrin-complexed honey with MGO levels of 400 mg/kg and 550 mg/kg had greater efficacy than that containing 250 mg/kg, but no difference was found to exist between the 400 mg/kg and 550 mg/kg levels.
Relative AUC for S. aureus ATCC 6838, MIC and MBC for S. aureus ATCC 6838, S. epidermidis 14990, and P. aeruginosa ATCC 27853 of cyclodextrin-complexed MGO Manuka Honey, uncomplexed Manuka honey and cyclodextrin. Relative AUC data are presented as mean±SD. Asterisks denote statistically significant differences (p<0.05).
As cyclodextrin-complexed Manuka honey and uncomplexed Manuka honey with an MGO level of 250 mg/kg exhibited antibacterial activity in the time course experiment, MGO levels under 250 mg/kg were subsequently assessed for their bacteriostatic and bactericidal effects with BHI broth serving as a control.
Bacterial growth patterns were consistent between all replicates of each assay for determining MIC and minimum bactericidal concentration. Cyclodextrin-complexed Manuka honey exhibited greater inhibitory and bactericidal actions on S. aureus and S. epidermidis than uncomplexed honey, although antibacterial effects against P. aeruginosa were comparable. At the levels assayed, no inhibitory or bactericidal actions were observed with cyclodextrin alone, while uncomplexed honey did not exhibit any bactericidal effects, and cyclodextrin-complexed Manuka honey was not bactericidal towards P. aeruginosa.
Taking into account the results of the AUC evaluations, as well as the commercial availability of the different concentrations of the cyclodextrin-complexed Manuka honey, the 400 mg/kg MGO concentration of cyclodextrin-complexed Manuka honey was selected for further evaluation.
Formulation preparation and in vitro human corneal epithelial cell viability evaluation
After addition of the MGO Manuka Honey complex, the clear microemulsion underwent phase transition into a liquid crystalline semisolid suitable for application to the eyelids. Gamma irradiation had no significant effect on the physical stability, pH and osmolarity (6.29±0.25 and 603.4±10.9 mOsm/kg, respectively; 1:10 in PBS).
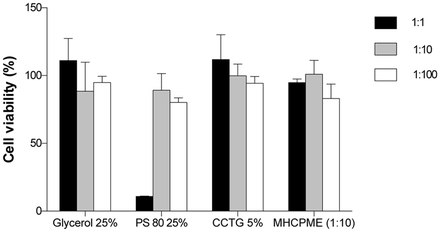
HCEC did not show significant toxicity following 15 min application of formulation excipients or the final MHME (figure 1) with the exception of the 1:1 dilution of PS 80 (cell viability 10.95%±0.26%). However, application of a concentration of surfactant equivalent to that incorporated in the final formulation proved to be non-toxic (cell viability >95%).
In vitro percentage cell viability following application of formulation excipients and the final MHME (1:10 dilution) to human corneal epithelial cells for 15 min. Each bar represents the mean percentage cell viability. Error bars represent the SD. CCTG, caprylic/capric triglyceride MI12; MHME, Manuka honey microemulsion; PS 80, polysorbate 80; SD, standard deviation.
In vivo phase
Paired-eye tolerability evaluation
Table 2 illustrates the summary statistics of the in vivo rabbit paired-eye tolerability evaluation. There were no significant differences in baseline tear film and ocular surface measurements between the eyes instilled with diluted MHME or saline control (all p>0.05). RM-ANOVA demonstrated no significant treatment, time, or treatment-by-time effects for any of parameters assessed during the 5-day period (all p>0.05). Post hoc multiplicity-adjusted Sidak's analysis showed that there were no immediate treatment effects following instillation of diluted MHME or saline control at all time points assessed (all p>0.05), and no significant cumulative treatment effects were observed during the trial period (all p>0.05).
In vivo tear film parameters and ocular surface characteristics during the 5-week trial period of the eyes of rabbits instilled with undiluted MHME (1:10) or saline control. Data are presented as median (range) or mean±SD. Asterisks denote statistically significant differences (p<0.05).
Tear film osmolarity profile evaluation
Baseline tear film osmolarity did not differ significantly between the eyes instilled with diluted MHME or saline control (p=0.99, figure 2). RM-ANOVA showed that there were no significant treatment, time, or treatment by time interaction effects for tear film osmolarity during the 10 min period following instillation (all p>0.05). Multiplicity-adjusted Sidak's post hoc analysis did not detect significant treatment effects at any of the time points assessed (all p>0.05).
In vivo tear film osmolarity profile during the 10 min period following instillation of diluted MHME (1:10) or saline control to rabbit eyes. Each point represents the mean tear film osmolarity at a given time point: solid circles represent eyes instilled with diluted MHME, and hollow circles represent eyes instilled with saline control. Error bars represent the SD. Post hoc multiplicity-adjusted Sidak p values of the treatment effects at each time point are provided above the error bars. MHME, Manuka honey microemulsion; SD, standard deviation.
Undiluted formulation safety evaluation
Summary statistics of the in vivo undiluted formulation safety evaluation are illustrated in table 3. No significant differences in baseline ocular surface characteristics were observed between the eyes instilled with diluted MHME or saline control.
In vivo ocular surface characteristics during the 10 min period following instillation of undiluted MHME (1:10) or saline control to rabbit eyes. Data are presented as median (range). Asterisks denote statistically significant differences (p<0.05).
Significant treatment, time, and treatment-by-time interaction effects for conjunctival hyperaemia grade were observed (all p<0.01). Post hoc multiplicity-adjusted Sidak's analysis detected elevated conjunctival hyperaemia grades relative to baseline in the MHME group at 30 s and 5 min (both p<0.001), and these values were significantly greater than the saline control group (both p<0.001). At 10 min, conjunctival hyperaemia in the MHME group decreased to levels that were not significantly different from baseline (p=0.37), and there were no differences between the MHME and saline control groups (p=0.85). No significant treatment, time, and treatment-by-time interaction effects for corneal opacity, iris appearance or fluorescein staining were detected (all p>0.05).
Discussion
The in vitro phase demonstrated antimicrobial activity of both cyclodextrin-complexed and uncomplexed MGO Manuka Honey solutions against bacterial species commonly associated with blepharitis, following pretreatment with catalase. The neutralisation of hydrogen peroxide by catalase treatment indicates that the bioactivity is attributable to other constituents including MGO, which has previously been identified as a significant antimicrobial factor in Manuka honey.21 25 MGO is believed to exert its effects via inhibition of murein hydrolase which is involved in the breakdown of peptidoglycan during cellular division.26 It is conceivable that with less peptidoglycan,27 and thus less murein hydrolase, Gram-negative organisms may be less susceptible to the antimicrobial effects of MGO than Gram-positive bacteria.28 This was demonstrated by the stronger sensitivity of S. aureus and S. epidermidis towards MGO than P. aeruginosa in the current study, and is consistent with previous studies which also report discrepancies between the response to Gram-positive and Gram-negative organisms.20 29 30 Nevertheless, blepharitis is more commonly associated with overcolonisation of S. aureus and S. epidermidis,6 against which MGO Manuka Honey solutions exhibited greater activity. It is also acknowledged that laboratory-adapted bacterial strains, used in our in vitro testing, have the potential to behave differently from clinical strains.31
Cyclodextrin-complexed Manuka honey solutions were generally shown to have stronger antimicrobial effect than uncomplexed honey, with smaller relative AUCs being observed in cultures containing cyclodextrin-complexed Manuka honey than uncomplexed honey. The magnitude of this effect paralleled the concentration of MGO. These findings are consistent with the previously reported enhancement of the antibacterial properties of Manuka honey following complexation with cyclodextrin.20 This is thought to be attributable to the slowed and sustained release of active honey constituents, and thereby the maintenance of an inhibitory concentration of these factors for an extended time.20 Complexation to cyclodextrin has been shown to reduce the release rate of many hydrophobic drugs to sustain a therapeutic concentration.18 Furthermore, in agreement with previously reported findings,20 cyclodextrin, alone, exhibited some antibacterial activity in the current study. However, these effects were significantly lower than those displayed by cyclodextrin-complexed or uncomplexed Manuka honey regardless of MGO concentration. Nevertheless, this suggests that the enhanced antimicrobial activity in cyclodextrin-complexed honey may be attributable to a combination of the complexation reaction and synergistic effects.
A microemulsion similar to that developed and described here has previously been reported to undergo phase transition from a clear liquid to a semisolid liquid crystal upon reduction of the glycerol and/or Tween 80 content.32 As cyclodextrins are capable of forming complexes with many excipients including glycerol,33 and Tween 80,34 it is likely that cyclodextrins from MGO Manuka Honey complexed glycerol and/or PS 80 leading to a component ratio shift and thus phase transition. This complexation behaviour is also supported by the cell viability data with death of almost 90% of cells exposed to PS 80 (1:1 dilution) for 15 min while PS 80 incorporated into the final formulation, and thus complexed and less available to the environment, caused no significant cytotoxicity. It should be noted here that, while the tested exposure time of 15 min is short, any formulation components accidentally entering the eye would be washed away quickly by reflex tearing and rapid tear turnover thus concentrations tested here are unlikely ever to be reached. Overall, formulations were found suitable for eyelid application with no anticipated significant ocular adverse effects in the event of formulation components entering the eye across the lid margins.
The subsequent in vivo phase, evaluating the safety and tolerability of the MHME in rabbit eyes, showed that measurements of lipid layer grade, tear evaporation rate, tear film osmolarity, phenol red thread, fluorescein staining, conjunctival hyperaemia, corneal opacity and iris appearance grades, did not change following instillation of either diluted MGO MHME or saline control. Furthermore, there were no significant changes in these measurements during the 5-day period in both groups, and no differences between groups. This suggests that instillation of diluted MHME was not associated with tear film destabilisation, ocular surface irritation, inflammation or epithelial damage in the rabbit eye. Furthermore, the MHME formulation is intended to be used as a cream applied onto the periocular skin of closed eyelids. However, this mode of application was not possible in the rabbit model due to fur around the eyes, and thus direct ocular surface instillation of an MHME at 1:10 dilution in saline was used in the current study. The migration of periocular particles into the tear film can occur in human subjects due to the action of the muscles of Riolan,35 36 and the surface tension at the tear meniscus.37 However, it is unlikely for the concentrations that reach the tear film with periocular application in humans to exceed the levels directly instilled into the rabbit eyes in the current study. Nevertheless, despite the favourable findings of this animal study, future tolerability trials on human subjects are required to confirm the clinical safety of MHME.
Although the MGO MHME is designed for topical application to the periocular skin of closed eyelids in human patients, the potential for accidental instillation directly onto the ocular surface cannot be discounted. Such a situation was simulated in the in vivo phase through the direct ocular instillation of undiluted MHME in the rabbits, without the immediate aqueous flushing that would be recommended as standard in clinical use following accidental instillation. Although the conjunctival hyperaemia grade was elevated at 30 s following undiluted MHME instillation, levels returned to baseline within 10 min.
The results of the in vivo phase need to be interpreted cautiously in the context of the methodological limitations, which preclude the direct extrapolation of the findings to the clinical safety and tolerability in human patients. There are significant differences in the anatomy and physiology of the eyes of rabbits and humans. Nevertheless, the rabbit is one of the closest models of the human ocular surface and tear film, and preclinical animal studies are required prior to the conduct of clinical trials in human subjects.
Of note, cyclodextrin-complexed Manuka honey generally exhibited stronger in vitro antimicrobial effects than uncomplexed honey on bacteria commonly associated with blepharitis. In vivo safety and tolerability evaluation of MGO MHME on rabbit eyes did not result in significant immediate or cumulative adverse effects. These findings therefore support clinical safety and tolerability evaluation of MGO MHME in human subjects.
References
Footnotes
Acknowledgements The authors are grateful to Angela Cunningham, BSc; Yen-Heng (Amy) Chen, BOptom; May Young, BOptom; Iana Pearce, BOptom; and William Perriam, BOptom, for their involvement in preliminary aspects of the work reported in this manuscript.
Contributors All authors took an active part in the design, conduct, data analysis, and publication drafting and approval.
Funding This research received unrestricted grant funding from the Faculty of Medical and Health Sciences at the University of Auckland and from Manuka Health, who themselves received grant support from the Ministry of Business, Innovation and Employment (MBIE PROP31150VCHMANUKAHEALTH).
Competing interests None declared.
Ethics approval University of Auckland Animal Ethics Committee UAAEC 001156.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement All data relating to the study are published in the manuscript and approaches to share this can be made to the corresponding author. Queries relating to the MGO Manuka Honey microemulsion can be made to Manuka Health New Zealand.