Article Text
Abstract
Objective The objective of this study is to evaluate the efficacy of intravitreal ranibizumab (IVR) monotherapy compared with intravitreal bevacizumab (IVB) monotherapy for treatment of type 1 and aggressive retinopathy of prematurity (ROP) in rural Egypt.
Methods 36 eyes of 18 infants with bilateral aggressive or type 1 ROP were recruited between September 2020 and September 2022. Mean follow-up duration was 16.53 months. IVB was injected in the right eye and IVR in the left eye, rescue injection of the same initial anti-vascular endothelial growth factor (VEGF) in case of ROP reactivation. Outcome measures included regression achieved either by single injection or multiple injections or additional laser therapy at 55 weeks’ postmenstrual age (PMA), recurrence of ROP, total retinal vascularisation time and complications.
Results Initial regression of ROP within 1 week occurred in 11/18 eyes (61.1%) in bevacizumab group and 15/18 eyes (83.3%) in ranibizumab group (p=0.137). Primary outcome measure was achieved in 14/18 eyes (77.8%) and 16/18 eyes (88.9%) in bevacizumab and ranibizumab groups, respectively (p=0.658). Late reactivation requiring retreatment with anti-VEGF was encountered in 4/18 eyes (22.2%) and 1/18 eyes (5.6%) in bevacizumab and ranibizumab groups, respectively (p=0.338). Peripheral laser therapy on the avascular retina was done in 3/18 eyes (16.7%) in each group at mean of 55.67 weeks' PMA.
Conclusion Bevacizumab and ranibizumab proved to be effective regarding regression of acute ROP and continuing peripheral retinal vascularisation. Higher proportion of reactivation with bevacizumab, however, clinically non-significant. Laser therapy can be postponed to reduce its complications.
Trial registration number NCT05033106.
- vision
- retina
- neovascularisation
- child health (paediatrics)
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Ranibizumab is the only approved anti-vascular endothelial growth factor (VEGF) for treatment of retinopathy of prematurity (ROP); however, some studies have reported that recurrence is much more common with ranibizumab than with bevacizumab.
WHAT THIS STUDY ADDS
In this prospective study, we concluded that both bevacizumab and ranibizumab can effectively reduce acute-phase ROP with no significant difference between the two drugs as regard recurrence necessitating retreatment.
Reinjection of anti-VEGF is recommended for ROP reactivation even in aggressive cases, so laser treatment can be reduced or postponed to avoid its complications.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The current study demonstrated a suggested algorithm that proved to be effective regarding initial treatment and treatment of recurrence for type 1 and aggressive ROP in rural Egypt preterm infants.
How should healthcare authorities approach the off label status of a potentially cost-saving therapy, bevacizumab, for this vision-threatening disease, ROP?
Introduction
Retinopathy of prematurity (ROP) remains a leading cause of preventable childhood blindness specifically in developing countries because of inappropriate neonatal care and lack of ROP screening programmes.1 It is a vascular endothelial growth factor (VEGF)-driven vasoproliferative disease.2
Over the past decades, photocoagulation of the retina by laser was the gold standard in treating ROP.3 Laser therapy exhibited an approximately 10% risk of retinal detachment in Early Treatment ROP (ETROP) randomised trial.4 Anti-VEGF treatment allows normal vascularisation of peripheral retina, better refractive outcomes as well as short duration of the procedure, easy application of the injection and avoidance of the risks of general anaesthesia.5
Bevacizumab is a humanised full-length monoclonal antibody that binds to all VEGF isoforms. Bevacizumab molecule is approximately three times larger than ranibizumab, and its higher molecular weight results in an intravitreal half-life that is 36% higher than that of ranibizumab. Ranibizumab is a humanised recombinant antibody fragment and has nearly 10 times greater affinity for VEGF.6 Ranibizumab is the first approved anti-VEGF treatment for the management of retinopathy and is a promising alternative to laser therapy. High treatment success rates were observed with ranibizumab 0.2 mg during the RAINBOW trial, with supporting evidence provided by the CARE-ROP trial.7 Ranibizumab is much more expensive than bevacizumab.8
VEGF is vital in angiogenesis, maintaining organ health and development of various vital organs in the body.9 The inhibition of VEGF may lead to abnormal organogenesis or neurodevelopment. The selection of an anti-VEGF drug with less systemic VEGF interference, reducing the dose or using an anti-VEGF agent only once in ROP patients seems to be safer. Intravitreal ranibizumab (IVR) was perceived as being a safer drug in premature infants due to the shorter duration of systemic VEGF suppression compared with intravitreal bevacizumab (IVB).10 Despite the increasingly widespread use of anti-VEGF agents in the treatment of ROP in recent years, information about their systemic effects and side effects is limited.11
It was reported that IVB and IVR have similar treatment efficacies but relatively higher disease recurrence following IVR therapy.12 Late recurrence at up to 70 weeks’ postmenstrual age (PMA) after IVB monotherapy has been also reported.13 So, timely detection and management of recurrence have become a major issue in anti-VEGF therapy for ROP.
Vitrectomy and/or scleral buckling (SB) are considered for stages 4 and 5 ROP. The reattachment rates with SB in stage 4A were 66%–75%,14–16 however, recently approached 93.5%.17 18 Lens sparing vitrectomy for stage 4A ROP has reported anatomical and functional success rates that ranged from 77% to 97%.19–21 The anatomical success for stage 4B was only 44.4%.22 The rates of reattachment for stage 5 ROP have been reported as disappointing, ranging between 13% and 45.5%.23 24
In this prospective study, we compared the efficacy of IVR and IVB for type 1 and aggressive ROP (A-ROP), as regard acute ROP regression, recurrence profile, needed additional treatment, complications, retinal vascularisation time and necessity of subsequent ablative procedures.
Methods
This prospective parallel assignment, single masking (participant) randomised clinical trial was approved by the ethical committee of Cairo University and Institutional Review Board Zagazig University. The study protocol was registered on www.clinicaltrials.gov.
The sample size was calculated to be 36 eyes of 18 infants using open Epi confidence total 95%, power of the study 80%.25 The right eye was assigned to receive bevacizumab and the left eye to receive ranibizumab by simple random method (by lottery) at the beginning of the study. Moreover, in our study group, both infants’ eyes were nearly of the same disease severity. Enrolled eyes were allocated in 1:1 ratio. Consort flowchart and checklist are provided in online supplemental files 1 and 2. The full trial protocol is provided as online supplemental file 3.
Supplemental material
Supplemental material
Supplemental material
We informed the participants’ parents about the severity of disease, treatment options and complications. Written informed consent was signed.
ROP screening was done by binocular indirect ophthalmoscope (BIO) and wide-field paediatric retinal imaging system (RetCam; Clarity Medical Systems, Pleasanton, California) according to ‘American academy of paediatrics recommendations’.26 Inclusion criteria: Infants with type 1 ROP and A-ROP affecting both eyes. Type 1 ROP, as defined by the ETROP study,4 is zone I ROP with plus disease, zone I stage 3 ROP without plus disease or zone II stage 2 or 3 ROP with plus disease. The hallmark of A-ROP (previously known as AP-ROP) is rapid development of pathological neovascularization and severe plus disease without progression being observed through the typical stages of ROP. It may occur in larger preterm infants and beyond the posterior retina.27 Exclusion criteria: eyes with previous intravitreal injections (IVIs) or laser therapy, eyes with any pathology other than ROP and eyes with ROP stage 4 or 5. Each infant was examined by an experienced paediatric ophthalmologist and a vitreoretinal surgeon independently.
Intravitreal injection (IVI) was performed within 72 hours once treatment criteria were confirmed. It was done under topical anaesthesia in standard ophthalmic operating room. Either 0.625 mg/0.025 mL bevacizumab or 0.25 mg/0.025 mL ranibizumab was injected intravitreally with a 31-gauge needle, aiming directly towards the optic nerve in direction of visual axis, at 1.0 mm posterior to the corneoscleral limbus. The IOP was checked postinjection by indirect ophthalmoscopic examination of the optic disc perfusion and central retinal artery pulsation. Topical antibiotics were given for 7 days postoperatively. Infants were examined on the next day then weekly until regression of ROP, then every (2–4) weeks until a minimum of 55 weeks’ PMA or when retinal vascularisation reached zone III without an active component such as haemorrhage or clinically significant tractional elements, whichever came earlier. Follow-up was continued monthly for at least 6 months following treatment.
Failure of initial regresssion was defined as persistence of plus disease and/or neovascularisation at 3–5 days’ postinjection.7 We wait for a maximum of 5 days to state ‘Failure of initial regression’. However, reactivation/recurrence (disease regression followed by reappearance of preplus or plus disease, extraretinal new vessels or fibrovascular ridge)27 can occur at any time throughout the follow-up period of 55-week’ PMA.
Both failure of initial plus regression or ROP recurrence were managed by rescue therapy injection of the same initial anti-VEGF.
In case of failed retinal vascularisation to approach zone III until 55 weeks’ PMA, an indirect infrared diode laser (IRIDEX, Iris Medical SL laser with laser indirect ophthalmoscope Ophthalmic Laser, 810 nm, USA) was used to apply photocoagulation to the peripheral avascular retina through a +22/+28 diopter condensing lens under general anaesthesia in the operating room. Retinal vascularisation was judged by both clinical examination by BIO and Retcam fundus photography at baseline and throughout the follow-up visits.
Primary outcome measures
The number of eyes achieved regression of active ROP either by single or two injections or additional laser therapy at 55 weeks’ PMA and total time till full retinal vascularisation (within two disc diameter (DD) from the ora serrata).
Secondary outcome measures
The number of eyes with recurrence of ROP requiring retreatment before 55 weeks’ PMA, the number of eyes that needed late peripheral laser and the number of eyes progressing to stage 4 or 5 necessitating vitrectomy with/without lensectomy.
Statistical analysis
We coded the data using the SPSS V.28 (IBM, Armonk, New York) and summarised it using mean, SD, minimum and maximum for quantitative variables, frequencies (number of cases) and relative frequencies (percentages) for categorical variables. Unpaired t-test was used to compare groups. χ2 test was performed to compare categorical data. Exact test was used instead when the expected frequency is less than 5. P value less than 0.05 meant a statistically significant difference.
Results
A total of 36 eyes of 18 infants were divided into two groups: (1) IVR group: included 18 eyes of 18 infants and (2) IVB group included 18 contralateral eyes of the same 18 infants. No significant difference between the two groups was noticed as regard demographic characteristics, duration of stay in neonatal intensive care units (NICU) and systemic condition (table 1). Patients were recruited between September 2020 and September 2022. The mean follow-up after the first procedure was 16.53 months (ranged from 12.1 to 23.8 months). The mean initial treatment time was 37 weeks’ PMA (ranged from 33 to 41 weeks).
Demographic characteristics of patients in the study
We defined treatment success as complete regression of the retinopathy with single IVI at 55 weeks’ PMA and vascularisation reached zone III without any additional treatment.
In IVB group, treatment success was achieved in 14/18 eyes (77.8%). Meanwhile, in the IVR group, 16/18 eyes (88.9%) achieved the target (p=0.658). Vascularisation approached zone III in 16/18 eyes (88.9%) in both IVB and IVR groups (p=1) (table 2). Mean PMA when maximum vascularisation occurred was 51.11 weeks (table 3).
The main ocular data in both IVR and IVB groups
PMA (in weeks) of the infants in both IVR and IVB groups at time of interventions
In IVB group, initial regression of neovascularization and plus disease was achieved within 1 week in 11/18 eyes (61.1%). In IVR group, this was achieved in 15/18 eyes (83.3%) (p=0.137). Failure of initial plus regression, that necessitated reinjection, occurred in 3/18 eyes (16.7%) in IVB group. On the contrary, no eyes in IVR group showed failure of initial plus regression (p=0.229).
Rescue therapy injection was defined as the need for a second dose of same initial anti-VEGF after the first injection and before 55 weeks’ PMA. Time interval between initial IVI and rescue therapy injection ranged from 1 to 3 weeks in cases of failure of initial regression and 12to 13 weeks in case of recurrence.
As regard IVB group, 4/18 eyes (22.2%) needed rescue therapy injection (three eyes for failed initial regression of acute ROP as shown in figure 1 and eye for late reactivation). Meanwhile, in the IVR group, 1/18 eyes (5.6%) developed late reactivation. No significant difference was found between the two groups (p=0.338). The mean PMA of reactivation of ROP in IVB group was 40.75 weeks. While in IVR group, it was 48 weeks (p=0.305). The mean initial treatment to reactivation interval was shorter in IVB group (4.25 weeks) than in IVR group (13 weeks) (p=0.233). Demographic and ocular data for infants/eyes that needed rescue therapy injection are shown in table 4.
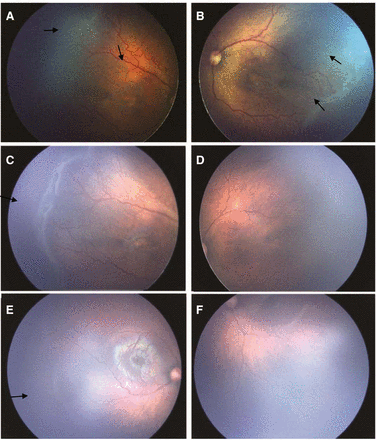
Retcam fundus photographs of a female baby (GA 28 weeks, BW 1200 grams). (A)Temporal fundus photograph of the right eye obtained at 36 weeks’ PMA before IVB showed type 1 ROP with severe plus disease in zone I, stage 3. (B) Temporal fundus photograph of the left eye before IVR showed A-ROP with plus disease in zone I, stage 3, double ridge, retinal neovascularisation. (C) Temporal fundus photograph of the right eye, obtained one week after IVB, showed failed initial plus regression, even aggravated plus disease, extensive retinal neovascularisation, aggravated ridge and arteriovenous shunts. (D) Temporal fundus photograph of the left eye, obtained one week after IVR, showed better initial plus disease regression, regression of a ridge and retinal neovessels completely. (E) Temporal fundus photograph of the right eye 49 weeks’ PMA, after reinjection of avastin showed faint line at the site of old ridge with vessels crossing it to the periphery, continued anterior retinal vascularisation. (F) Inferotemporal fundus photograph of the left eye at 49 weeks’ PMA showed disappearance of active ROP, but still avascular retina at anterior zone II. GA, gestational age; BW, birth weight; IVB, intravitreal bevacizumab; IVR, intravitreal ranibizumab; PMA, postmenstrual age; ROP, retinopathy of prematurity.
Demographic and ocular data for eyes that needed rescue therapy injection in IVR, IVB groups
Late laser therapy on the peripheral avascular retina was needed in 3/18 eyes (16.7%) in both IVB and IVR groups at mean of 55.67 weeks’ PMA (range from 55 to 57 weeks).
After initial IVI, no infant had intraocular heamorrhage or endophthalmitis. One infant in IVR group developed subconjunctival haematoma that totally resolved after 2 weeks. Regressed neovascularisation caused localised preretinal haemorrhage in four eyes in each group. These haemorrhages resorbed within 3 weeks without any sequalae. An infant in IVB group developed localised lens opacity in the right eye after the second IVB. It was away from the visual axis, did not affect fixation and did not progress till the end of follow-up period. Through the follow-up visits, no eyes showed progression to stage 4 or 5. In all infants, the macula was intact without macular fold, or foveal dragging.
As regard systemic adverse events, two infants showed delay in growth and motor milestones. One of them was diagnosed as hydrocephalus. He is still being followed in the neurosurgery clinic and the rehabilitation department, as he underwent repeated ventriculoperitoneal shunt surgeries. The other infant was diagnosed as spastic cerebral palsy of prematurity. We believe that these presentations are considered known complications of prematurity, therefore not associated with our intervention.
There was no correlation between the need for rescue therapy and BW, GA, PMA at initial treatment (p=0.249, p=0.658, p=0.627, respectively). 4/5 eyes, that needed rescue therapy, were treated with bevacizumab. But this did not reach statistical significance to correlate the type of drug injected to the need for rescue injection (p=0.338). A-ROP was found in 3/5 eyes, that needed rescue therapy, still no significant correlation between staging of ROP and the need for rescue injection (p=0.740). There was no statistically significant correlation between zone of ROP and the recurrence rate (p=1.0) as shown in table 2.
Discussion
The third epidemic of ROP mostly involves middle income countries, like Egypt where wider NICU availability is increasingly supporting the survival of infants, but suboptimal care and improper oxygen administration and monitoring are resulting in higher rates of ROP among older and heavier infants.25 28 This is especially true in rural settings where higher rates of more severe forms of the disease are reported.29 30 Intravitreal anti-VEGF agents are currently used as a first-line therapy for the treatment of ROP, rather than an adjunctive or supplemental therapy. Despite their ocular advantages, prolonged follow-up is required because of incomplete retinal vascularisation.31 In the current study, we prospectively reported the outcomes of 36 eyes of 18 infants with type 1 ROP (20 eyes) and A-ROP (16 eyes) who received IVB (18 eyes) and IVR (18 eyes). Every patient was his own control, which greatly minimised the effect of the systemic condition, gestational age (GA), birth weight (BW) and weight gain on the results.
We used a second IVI of the same initial anti-VEGF drug for ROP reactivation or failed regression of acute ROP. This helps to purely assess the effect of the two different anti-VEGF drugs without any overlap that may occur due to cross treatment32 or combined treatment.33 We believe that a second injection represents a better alternative to rescue laser photocoagulation34 for ROP recurrence.
Gunay et al35 collected the data of 134 infants (264 eyes) retrospectively. Type 1 ROP or AP-ROP cases received either IVB (55 infants), IVR (22 infants) or diode laser photocoagulation (57 infants). All eyes showed complete resolution of neovascularisation after single injection but recurrence of ROP occurred in 3 of 55 infants (5.5%) treated with IVB, 11 of 22 infants (50%) treated with IVR and 1 of 57 infants (1.7%) treated with laser photocoagulation. The mean time to recurrence after IVR was 8.8±1.5 weeks compared with 14±2.7 weeks with IVB. All infants with recurrence in IVB group required bilateral retreatment. While only 3% with recurrence in IVR group required bilateral retreatment, no difference in retreatment rates.
Unlike our study results in which 4/18 eyes (22.2%) needed reinjection in the IVB group. Meanwhile, 1/18 eyes (5.6%) developed late reactivation after IVR monotherapy. Failed initial plus regression in the IVB group in our study may be explained by deterioration of the intrinsic properties of the molecule or the potency of the drug as a result of the repackaging into plastic syringes36 or duration of the storage.37 Alliquoting of bevacizumab is challenging. It needs to be done with complete aseptic precautions by the compounding pharmacies. Even so, the risk of contamination, degradation of the molecule cannot be completely ruled out. In the absence of compounding pharmacies, the risk is higher.
Alyamac et al38 recorded retrospectively a review of 45 infants (90 eyes) with type 1 ROP-affecting zone I or posterior zone II. IVB group included 44 eyes and IVR group included 46 eyes. Recurrence occurred in 14/23 infants (61%) treated with ranibizumab and 6/22 infants (10%) treated with bevacizumab. 2/6 infants (33%) with recurrence in IVB group required laser photocoagulation was needed as additional treatment for 2/6 infants (33%) with recurrence in IVB group at 43 weeks’ PMA and 2/14 infants (14%) with recurrence in IVR group at 42.5 weeks’ PMA.
Although the reactivation rate in IVR group was much higher than IVB group according to Alyamac et al, the rescue therapy was needed at a higher rate in IVB group in partial agreement with our results. Laser photocoagulation was done for 33% in IVB group, which is higher rate than the current study, mostly because it was done earlier (43 vs 55.67 weeks’ PMA).
Alyamac et al38 revealed that complete retinal vascularisation was detected in the 55.9 weeks’ PMA in IVB group and in the 56.3 weeks’ PMA in IVR group. In the current study, the time of full retinal vascularisation was 51.11 weeks’ PMA in both groups. This 5-week difference is mostly due to the fact that total retinal vascularisation is defined in our study as perfusion within 2 DD from the ora serrata. However, according to Alyamac et al, it was defined as retinal vessels reaching the ora serrata.
Lin and Tsai39 noted that 15/25 eyes (60%) treated with ranibizumab and 7/15 eyes (47%) treated with bevacizumab showed complete retinal vascularisation. The authors assumed that the IVR could achieve more complete retinal vascularisation than IVB because IVR has shorter intravitreal VEGF suppression.39
Kimyon and Mete34 reported a higher recurrence rate (7.1%) than our study, although near similar GA and BW. Several studies with lower GA and BW revealed a higher reactivation rate (20.8%–83%).32 Other studies noted high reactivation rate despite average GA or BW.40 The lower reactivation rate in our study (5.6%) was mostly due to relatively mature infants (later GA and higher BW) than some other studies. Those infants of a younger GA and lower BW would have been more ill with a more serious ROP necessitating earlier intervention, so an early PMA at initial therapy is a risk factor for ROP reactivation.32
Kabatas et al41 analysed the reports of 54 infants (108 eyes) with type 1 ROP who received IVB (24 eyes), IVR (12 eyes) or diode photocoagulation (72 eyes) retrospectively. Recurrence occurred in 2/12 eyes (16%) treated with ranibizumab and 2/24 eyes (8.3%) treated with bevacizumab. According to Kabatas et al,41 complete vascularisation in IVB group was detected at 73±10.1 weeks’ PMA and 61.8±6.6 weeks’ PMA in IVR group.
The number of cases in IVB group is two times that in IVR, so the recurrence rate appeared higher in the latter although two eyes in both groups experienced recurrence of ROP. This explained the different results when compared with ours.
Unlike the current study, mean PMA when maximally vascularised in both IVR and IVB groups was 51.11 weeks. That was a shorter time than Kabatas et al41 mostly because 55.5% of our cases were of type 1 ROP-affecting zone II. While in Kabatas et al41 type 1 ROP, affecting zone I represented 22.2% of eyes in ranibizumab and bevacizumab groups. It is logic that more time is needed to achieve full vascularisation for zone I compared with zone II ROP.
Feng et al42 found that more aggressive forms of ROP at initial IVR injection were significantly correlated with ROP recurrence. Recurrence was detected in 67% eyes with APROP, 38% eyes with threshold ROP and 16% eyes with type 1 prethreshold ROP. Higher recurrence rates in APROP have been noted in premature infants treated with either IVR43 or IVB.35 This is in agreement with our results in which recurrence, that necessitated rescue therapy injection, was seen in 3/16 eyes (18.75%) with APROP, 2/20 eyes (10%) with type 1 ROP.
In the current study, the initial treatment to reactivation interval in IVR group was 13 weeks and 4.25 weeks in IVB group. Wong et al44 noted the shortest reactivation interval (5.9 weeks) after IVR, this might be due to smaller GA (23.48 weeks) and lower BW (620 g) in their study population. Zhang et al45 reported the longest reactivation interval of (12.62±7.93 weeks), and this may be attributed to using a higher dose of ranibizumab (0.3 mg in 0.03 mL).
In this study, it was obvious that IVR was associated with better initial regression of plus (less venous diameter, less arterial toursousity), regression of neovascularisation and straightening of closed vascular loops as early as few days after injection. Also, it promotes better and earlier growth of normal retinal vasculature towards the retinal periphery than IVB. This may be due to shorter intravitreal half-life of ranibizumab. It can theoretically decrease the supplementary laser spots needed and the subsequent destruction of peripheral visual fields, which might offer potential vision benefits.
Still our results were contradictory to previous studies that reported more and faster recurrence with IVR. Most of these studies have been conducted on European,46 Asian46 47 or American population.48 To our knowledge, few studies were conducted among African (Egyptian) infants. So, there may be different levels of VEGF expression, ROP severity and treatment responses in different ethnic groups.45 49
The strength of our study appears as all cases were treated in a prospective manner, minimising the risk of missing data or incomplete examinations. Moreover, all cases were evaluated before and after injection, so that even minute changes are detected and analysed using coloured RetCam-saved photos and clinical examinations by the indirect ophthalmoscopy.
In many studies which compare the two medications in infants with ROP, one group of patients received IVR and a different group received IVB. In this study, the patient was his own control, receiving one drug in one eye and the other drug in the contralateral eye. This greatly minimises the effect of the systemic condition, birth weight, gestational age and weight gain on our results.
Our study had a relatively small sample size. The follow-up period was enough to document ocular efficacy of the two drugs, but relatively short to document systemic safety. The study targeted the assessment of ocular efficacy of the two anti-VEGF drugs, of which one holds a clear price advantage. Ranibizumab (Lucentis) is up to 50 times more expensive than bevacizumab (Avastin)50 so health services in our developing countries could make significant savings by using bevacizumab (Avastin). Though fundus fluorescein angiography is a useful tool in observing retinal vascular morphology and development, it was not used in our study. Our study population characteristics of higher BW and older GA were different compared with the previous publications. So, the results cannot be generalised especially for highly developed countries.
Conclusion
The current study demonstrated a suggested algorithm that was effective regarding initial treatment and treatment of recurrence for type 1 and A-ROP in preterm infants in rural Egypt. Following this algorithm, only 5/36 eyes (13.89%) needed a second IVI of anti-VEGF drug. Also, only 6/36 eyes (16.7%) needed peripheral laser after 55 weeks’ PMA. This greatly minimised the peripheral visual field defect and myopic shift and allow for improvement of the general condition in a physically compromised preterm newborn. Still, there is a dire need for larger randomised trials that analyse risk versus benefit regarding repeat anti-VEGF or minimal peripheral laser treatment especially for ROP reactivation-affecting anterior zone II.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by 1-IRB, Faculty of Medicine, Zagazig University ZU-IRB#6269/22-7-20202—Research Ethical Committee D-9-2020. Participants gave informed consent to participate in the study before taking part.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors GMT, AME: data collection, examination and manuscript writing; EAS: data analysis, manuscript revision and editing; SAD; DHH: data extraction, manuscript revision and supervision of the study process; DHH: study design, data analysis and manuscript editing; AME: data analysis, interpretation and manuscript revision. GMT: responsible for the overall cotent as guarantor. All authors contributed to the article and approved the submitted version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer All methods were performed in adherence to the relevant guidelines and regulations.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.