Article Text
Abstract
Objective The English Diabetic Eye Screening (DES) programme recommends patients with M1 diabetic maculopathy to be referred to hospital eye services. DES uses flash fundus photography as the reference standard for maculopathy grading. We compared multicolour versus non-stereoscopic fundus photography at identifying M1 maculopathy, with spectral domain optical coherence tomography (SD-OCT) identifying macular thickening.
Methods and analysis This cross-sectional study included 345 patients with R1M1 referred from DES and reviewed in secondary care with fundus photographs, multicolour and SD-OCT. Maculopathy was graded based on DES exudate criteria on both multicolour and fundus photography in a blind fashion by two independent graders. Macular thickness was ascertained on SD-OCT.
Results Intergrader agreement on grading maculopathy using fundus photography (Cohen’s κ=0.91) and multicolour (Cohen’s κ=0.82) was ‘almost perfect’. Agreement between fundus photography and multicolour on grading maculopathy (Cohen’s κ=0.76) was ‘substantial’. Compared with fundus photography, multicolour had sensitivity of 87% (95% CI 81% to 93%) and specificity of 90% (95% CI 87% to 94%) in detecting M1 maculopathy. SD-OCT identified 84 eyes with macular thickening, 47 of which were graded as M0 by fundus photography. 5 eyes with exudates and severe macular oedema requiring urgent intervention were also missed on fundus photography but not on multicolour. Multicolour, when complemented by SD-OCT, did not miss any clinically significant macular oedema.
Conclusion Multicolour integrates synergistically in a single platform with SD-OCT providing effective monitoring of M1 diabetic maculopathy. The need for fundus photography is eliminated by multicolour/SD-OCT in dedicated R1M1 virtual clinics not requiring parallel diabetic retinopathy grading.
- diagnostic tests/investigation
- imaging
- macula
- retina
- treatment medical
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Diabetic retinopathy is the most common cause of sight loss among working age adults. The English NHS Diabetic Eye Screening (DES) programme recommends patients with M1 diabetic maculopathy, diagnosed using flash fundus photography, to be referred to hospital eye services.
What are the new findings?
This is a cross-sectional study comparing fundus photography (FP) with MultiColor (MC) integrated with SD-OCT in diagnosing M1 maculopathy. As compared to FP, MC has excellent sensitivity and specificity in diagnosing M1 maculopathy. Furthermore, MC integrates synergistically in a single platform with SD-OCT providing effective monitoring of M1 diabetic maculopathy, identifying potential patients requiring treatment with the modern pharmacologic agents such as vascular endothelial growth factor inhibitors (anti-VEGF), eliminating the need for FP in dedicated R1M1 virtual clinics not requiring parallel diabetic retinopathy grading.
How might these results change the focus of research or clinical practice?
The DES criteria for maculopathy grading using non-stereoscopic FP is becoming increasingly less relevant in an era of anti-VEGF where OCT is becoming more important in diagnosing diabetic maculopathy. This study does not provide a direct evaluation of MC imaging and FP within the DES programme.
Introduction
In the UK, up to one-third of adults with diabetes have concurrent diabetic eye disease.1 2 It is the most common cause of sight loss among working age adults.3 Therefore, the English National Health Service Diabetic Eye Screening (DES) programme offers diabetic eye screening for individuals with diabetes aged 12 years and over.3
Diabetic maculopathy manifesting as diabetic macular oedema (DMO) is the leading cause of blindness among the population with diabetes.4 The main pathophysiological factor is thought to be the disruption of blood–retina barrier secondary to long-term hyperglycaemic insult and inflammation, which leads to leakage of plasma into the neurosensory retina resulting in DMO and hard exudates in the macula.4 5 The hard exudates are composed of lipids and plasma proteins accumulating mainly at the outer plexiform layer.6–8
The DES defines the macula as the area of the retina confined by a circle with the fovea at its centre and touching the temporal edge of the optic disc.9 The DES recommends patients with M1 diabetic maculopathy to be referred to the hospital eye services (HES).3 The DES definition of M1 maculopathy includes any exudates within one disc diameter (1DD) of the fovea, retinal thickening within 1DD of the fovea or a group of exudates that covers an area that is greater than or equal to half the disc area, all within the macula.9 10
The DES uses flash colour and red-free fundus photography (FP) as the reference standard for maculopathy grading.11 Colour FP is an imaging modality which closely concords with clinical examination findings when using an ophthalmoscope or slit-lamp biomicroscope. However, in the past decade, different systems based on confocal scanning laser ophthalmoscopy (SLO) for fundus imaging have been developed producing ‘pseudo-colour’ composites. Examples of these systems include Optos wide-field SLO (Optos, Dunfermline, UK) using green (λ=532 nm) and red (λ=633 nm) laser wavelengths12; Nidek F-10 confocal SLO (Nidek, Fremont, California, USA) which uses infrared (λ=790 nm), red (λ=660 nm), green (λ=532 nm) and blue (λ=490 nm) laser wavelengths13; multicolour (MC) confocal SLO imaging (Heidelberg Engineering, Heidelberg, Germany) which uses three simultaneously acquired laser reflectance images using infrared (λ=815 nm), green (λ=518 nm) and blue (λ=486 nm) wavelengths.14 Fundus images obtained using systems such as MC can appear strikingly different when compared with the FP, however, some macular pathologies appear more distinct on the MC.14 15
The additional benefit of MC system is that it is integrated with spectral domain optical coherence tomography (SD-OCT) in a single platform. This potentially allows for a more comprehensive evaluation of diabetic maculopathy, based on detection of exudates and retinal thickening, when compared with non-stereoscopic FP alone. Indeed, there is a good correlation between macular fluorescein angiographic leakage and visual acuity with OCT features of diabetic maculopathy.16 17
In this study, first, we aim to compare MC versus FP at identifying M1 maculopathy based on presence of macular exudates. Second, we endeavour to identify M1 maculopathy based on retinal thickening with SD-OCT, which is undetectable on non-stereoscopic FP. Finally, we compare the merits of integration of MC and SD-OCT versus FP when evaluating M1 maculopathy in dedicated R1M1 virtual clinics not requiring parallel diabetic retinopathy grading.
Method
Population and image acquisition
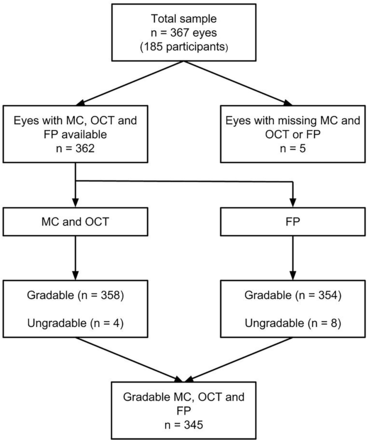
This is a cross-sectional study of 187 patients with diabetes (367 eyes) who were monitored in virtual macular clinics between 1 January 2012 and 1 January 2013 after an initial referral from the English DES programme to HES for further evaluation of R1M1 grading (figure 1). Mydriasis was achieved 30 min after instillation of preservative-free tropicamide 1% and phenylephrine 2.5% eye drops. A Zeiss Visucam Pro NM non-mydriatic fundus camera was used to obtain FPs consisting of 30° and 45° colour and red-free images centred on the fovea. Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) was used to acquire 30° MC images centred on the fovea as well as SD-OCT scans. Specifically, we used the built-in Fast Macula protocol of 20°×15° 6×6 mm volume centred on the fovea, consisting of 19 lines spaced 240 µm of 512 A-Scans each.
Flowchart of study participants. FP, fundus photography; MC, multicolour; OCT, optical coherence tomography.
Retinal grading
Exudate criteria
FP images were viewed using Zeiss VISUPAC software with its available image manipulation tools applied if needed. MC (including the individual blue, green and infrared reflectance images) and SD-OCT images were viewed using Heidelberg Eye Explorer with its image manipulation tools used as necessary.
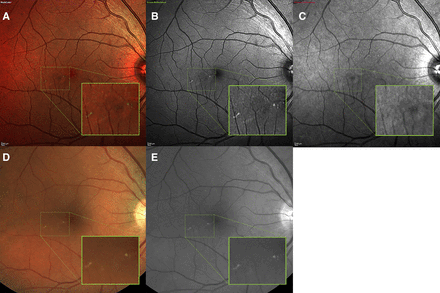
The MC and FP images were delinked. The diabetic maculopathy was graded based on exudate criteria as described by the DES on both MC and FP in a blind fashion independently by two graders (OK and SP). Infrared reflectance, green reflectance, blue reflectance and MC composite were assessed individually to decide on the presence of exudates (figure 2). An image was deemed ungradable if the detail of the third order retinal vessels from the superior and inferior vascular arcades was not discernible.
Visualisation of exudates on different imaging modalities. Macular exudates on multicolour composite (A), green reflectance channel (B), infrared reflectance channel (C), fundus photography (FP) (D) and red-free FP (E). The green reflectance channel is the main contributor to exudate visualisation on the multicolour composite image. Please note the absence of exudate on infrared reflectance channel as it images deeper retinal structures.
On SD-OCT, an exudate was defined as a homogenous hyper-reflective focus of ≥63 µm at, or internal to the outer plexiform layer with no inner hyporeflectivity. M1 maculopathy based on exudate criteria as outlined by the DES was not assessed systematically using SD-OCT as the spacing between the B-scans was >63 µm and the scan did not cover the entire macular as defined by the DES. However, any exudate seen on SD-OCT was recorded and correlated with FP and MC findings.
Macular thickness criteria
Macular oedema and thickness were ascertained on SD-OCT and retinal thickness maps on Heidelberg Eye Explorer by two independent graders (OK and SP). The thickness map was centred at the fovea and the retinal boundaries were defined between the internal limiting membrane and the Bruch’s membrane/retinal pigment epithelium complex—correction of the automated segmentation was made if needed. We defined abnormally increased macular thickening as central subfield thickness (CST) of ≥320 µm for men and ≥310 µm for women or thickening of ≥360 µm in any of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid inner sectors on Heidelberg Spectralis SD-OCT as described elsewhere.18 19 ‘Clinically significant macular oedema’ (CSMO) on SD-OCT was defined as spongiform or multicystoid retinal thickening of ≥370 µm in the CST or of >390 µm in any of the ETDRS grid inner sectors.19 A correction factor of +70 µm was used to convert Stratus OCT (Carl Zeiss, Oberkochen, Germany) reported values for retinal thickening in the literature to equivalent Heidelberg Spectralis SD-OCT.20 Eyes with other causes of increased macular thickness such as vitreomacular traction, epiretinal membrane and choroidal neovascularisation were excluded. Any disagreements between the two graders (OK and SP) were resolved by a third grader (MMDF).
Patient and public involvement
Patients were not directly involved in the design of this study.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, V.22. The tests were two tailed with type I error set at α=0.05. To ascertain the level of agreement between the two graders (OK vs SP) and the two different imaging modalities (MC vs FP), Cohen’s κ coefficient was calculated.21 FP was set as the reference standard and the sensitivity, the specificity, the positive predictive value and the negative predictive value of MC at detecting M1 maculopathy as per DES were calculated with 95% CIs.
Result
The study included 345 eyes with gradable images in all three modalities (figure 1). Either FP or MC image was missing from five eyes. There were four ungradable MC images and eight ungradable FP images. The rate of M1 diabetic maculopathy in this cohort was 32%, and 35% on FP and MC, respectively (table 1).
Clinical characteristics
Intergrader and intermodality level of agreement in detecting M1 diabetic maculopathy based on exudate criteria of DES
The levels of agreements are listed in table 2. There was an ‘almost perfect’ intergrader (OK vs SP) level of agreement in detecting M1 maculopathy on FP (Cohen’s κ=0.91; p<0.0001). There was a ‘substantial’ intergrader (OK vs SP) level of agreement in detecting M1 maculopathy on MC (Cohen’s κ=0.82; p<0.0001). The intermodality level of agreement between MC and FP in detecting M1 maculopathy was also ‘substantial’ (Cohen’s κ=0.76; p<0.0001 for OK and Cohen’s κ=0.72; p<0.0001 for SP). Furthermore, once the final grading for each patient was agreed on FP and MC, the intermodality level of agreement remained ‘substantial’ (Cohen’s κ=0.76; p<0.0001).
Intergraders and intermodality level of agreement grading M1 maculopathy as defined by DES
The diagnostic performance of MC in detecting M1 diabetic maculopathy based on exudate criteria of DES compared with FP as reference standard
Compared with the FP reference standard, the MC had sensitivity of 87% (95% CI 81% to 93%), specificity of 90% (95% CI 87% to 94%), positive predictive value of 80% (95% CI 73% to 88%) and negative predictive value of 94% (95% CI 91% to 97%) in detecting M1 maculopathy. In the peripheral macula, beyond 1DD of the fovea, where M1 is defined by group of exudates rather than any exudates, MC had sensitivity of 97% (95% CI 92% to 100%), specificity of 99% (95% CI 98% to 100%), positive predictive value of 95% (95% CI 87% to 100%) and negative predictive value of 99% (95% CI 99% to 100%) for detection of M1 maculopathy.
Macular thickening on SD-OCT
Twenty-four per cent (n=84) of eyes had a degree of foveal or parafoveal macular thickening based on criteria described above (table 1). Interestingly, 70% and 67% of eyes with M1 maculopathy on FP and MC, respectively, demonstrated no macular thickening on SD-OCT. Among the eyes with OCT macular thickening, 56% and 50% had no M1 maculopathy on FP and MC, respectively, and 44% of times had no M1 maculopathy either on FP or on MC.
CSMO based on SD-OCT
Eighteen eyes had CSMO on OCT, characterised by spongiform or cystoid retinal thickening causing CST ≥370 µm or thickening >390 µm of any of the ETDRS grid inner sectors (table 1). MC and FP did not detect M1 maculopathy in one (0.6%) and five eyes (28%), respectively.
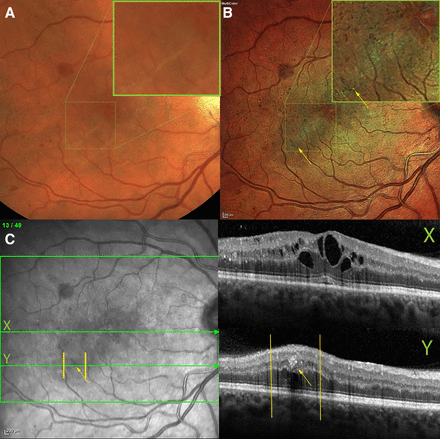
Five patients (1.4%) had a CST ≥400 µm, of whom only one had no M1 maculopathy on MC but four had no M1 maculopathy on FP (figure 3).
Macular exudate and thickening are evident on multicolour (B) and SD-OCT (C), but missing from fundus photo (A). There is a linearly shaped cluster of exudates on multicolour (B) that are missed on fundus photo (A), even at high magnification (inset). Two cross-sectional OCT scans are shown in (C), with its near infrared fundus registration image on the left: X, centred on the fovea, shows cystoid macular oedema; Y, is a line that cross-sections one of the exudates, marked with the yellow arrow, seen on the multicolour image (B) but not on the fundus photo (A) or on the infrared reflectance channel (C). SD-OCT, spectral domain optical coherence tomography.
Detection of exudates on SD-OCT
M1 maculopathy was not assessed systematically using SD-OCT. However, there were 19 cases of an exudate detected on SD-OCT within the 1DD of the macula, 10 of which were also detected on MC, but not on FP. In contrast, there were only three cases where an exudate was detected both on SD-OCT and FP but not on MC.
Discussion
In this study, we demonstrated that MC reliably detects M1 maculopathy with acceptable sensitivity and specificity, with a ‘substantial’ interobserver agreement, when compared with the current reference standard. Moreover, when synergistically integrated into a single platform with SD-OCT in a dedicated R1M1 virtual clinic not requiring parallel diabetic retinopathy grading, MC performs superior to FP in detecting and monitoring M1 maculopathy as described by DES.
Overall, the sensitivity and specificity of the MC are clinically acceptable and with a negative predictive value of 94% (95% CI 91% to 97%) in combined peripheral and central M1 maculopathy. However, when it comes to peripheral M1 maculopathy, beyond 1DD of the fovea, where DES criteria for M1 require the presence of discrete and grouped exudates, rather than any exudates, the MC sensitivity and specificity are excellent. Furthermore, MC has a comparable level of intergrader agreement to FP. Most of disagreements between MC and FP arose secondary to a small central exudate not being detected by one of the modalities due to the exudate being singular and small. In the case of MC, it could be due to its confocal nature and its thin focusing plane missing the small exudate. However, the group of exudates at the periphery was almost always detected by both of the modalities. Nevertheless, it must be remembered that FP, which is currently the reference standard for detecting M1 maculopathy within the DES, can miss exudates as identified on SD-OCT as shown here and elsewhere.14 Therefore, the true sensitivity and specificity of MC in detecting M1 maculopathy based on exudates criteria are unknown and may be redefined by OCT.
One of the most pertinent issues that arose in this study was the fact that more than two-thirds of the patients with M1 maculopathy according to exudate criteria on FP or MC had no macular thickening on SD-OCT. Conversely, one-fifth of patients with no signs of maculopathy on non-stereoscopic FP or MC had some degree of macular thickening based on SD-OCT. Perhaps more worryingly, 5 out of 18 patients with CSMO were missed by FP, whereas only one was missed on MC. Missing CSMO undermines the utility of FP as a screening tool for detection of maculopathy.
After the landmark ETDRS,22 grid/focal laser photocoagulation had been the first-line therapy for DMO for a number of years. However, since the advent of new pharmacological therapeutics in the form of intravitreal anti-vascular endothelial growth factor (anti-VEGF), the laser photocoagulation has been largely superseded by anti-VEGF therapy as the first-line therapy for central involving DMO.23 24
In the era of anti-VEGF therapy for DMO, along with visual acuity, the central retinal thickness is one of the most important quantitative factors considered for treatment decision-making in the majority of clinical trials.24 This renders the current DES grading criteria for definition of diabetic maculopathy based on central exudates with limited utility. In fact, the National Institute for Health and Care Excellence (NICE) guidelines for the use of anti-VEGF for DMO do not take into account the presence of exudates.25 26 Worryingly, the FP failed to identify a significant number of patients with CSMO. Worryingly, of the five individuals who met the CST criteria for treatment of DMO with anti-VEGF as per NICE guidelines,25 26 only four were identified as having M1 maculopathy based on FP.
In our study, while 32% of the 345 eyes assessed in this study had M1 grading by FP criteria, only 1.4% met the criteria for anti-VEGF therapy as outlined by NICE guidelines. The prevalence of diabetic maculopathy is increasing in the UK.27 28 The current DES-based classification and referral pathway for diabetic maculopathy may need refinement to adapt to the anti-VEGF era. If multimodality imaging inclusive of OCT is introduced at the point of referral from screening and applied in any subsequent monitoring scheme, patients may be spared unnecessary reviews in secondary care and be safely discharged back from the HES to the DES programme.
Although singular central exudates on their own are largely irrelevant in terms of treatment decision-making for DMO, detection of peripheral group of exudates may be important in treatment decision-making. This is the group MC had very high positive and negative predictive values for. These patients may not have central involving DMO to be eligible for anti-VEGF treatment but they can benefit from focal laser photocoagulation.24 29 The relative indication for focal laser can be considered in focally grouped microaneurysms and leaking capillaries, which may delay or prevent conversion into central involving DMO.24
A weakness of this study is that it does not provide a direct evaluation of MC imaging and FP within the DES programme. Our non-mydriatic fundus camera is a different model of the cameras used by our local DES programme. Moreover, simultaneous MC and FP grading would be required at the point of screening. Several patients monitored in the HES diabetic maculopathy virtual clinics had a considerable time lapse from the initial DES referral. The unexpected low prevalence of eyes with M1 grading in these clinics, while reflecting inadequate discharge policies, provided the strength for MC false-positive testing in our study.
In conclusion, the sensitivity and specificity of MC are comparable with that of FP in detecting M1 maculopathy, especially where peripheral group of exudates is concerned. The relevance of central exudates causing M1 maculopathy as detected by FP is largely diminished in the era of anti-VEGF therapy. Furthermore, FP can dangerously miss DMO and overburdens the secondary care with stable patients who can be safely followed up by DES. Detection of peripheral group of exudates not causing central involving DMO may still be relevant, as focal laser photocoagulation of leaking microaneurysms and capillaries may reduce the chance of central involving DMO. MC only detects peripheral group of exudates reliably but integrates synergistically in a single platform with SD-OCT to provide effective monitoring of M1 diabetic maculopathy. The need of FP is eliminated by MC/SD-OCT in dedicated R1M1 virtual clinics not requiring parallel diabetic retinopathy grading. Integrated MC/SD-OCT has the potential to reduce the burden on the overstretched secondary care by reducing the number of patients who do not require any treatment for their diabetic maculopathy and who can be safely followed up in the community.
Acknowledgments
We would like to thank the staff of the Department of Ophthalmology, University Hospitals Coventry and Warwickshire, Coventry, UK for their help.
References
Footnotes
Contributors OK designed the study, collected and analysed the data, and wrote the manuscript. MMDF collected the data and critically appraised the manuscript. MC collected the data and critically appraised the manuscript. EC captured the images and critically appraised the manuscript. SP designed the study, collected the data and critically appraised the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MC is paid advisor to Heidelberg Engineering, Zeiss, Alcon and Centervue. SP is paid advisor to Heidelberg Engineering, Zeiss, Novartis, Bayer and Allergan.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval This project was registered with the University Hospitals Coventry and Warwickshire NHS Trust Research, Development and Innovation Department. The Trust considered the work to be a departmental service evaluation, and as such, advised us that it did not require research ethics approval.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon request.