Article Text
Abstract
Objective We investigated the detailed time course of conjunctival hyperemia induced by omidenepag isopropyl ophthalmic solution 0.002% (omidenepag), a selective prostaglandin E2 receptor 2 agonist.
Methods and analysis We recruited 34 healthy subjects and administered omidenepag in the right eye and ripasudil 0.4% in the left eye. We evaluated conjunctival hyperemia using slit-lamp photography at baseline and after 15, 30, 60, 120, 180 and 360 min. The conjunctival hyperemia score was graded by three independent observers using a scale from 0 (none) to 3 (severe). We also evaluated conjunctival hyperemia by the pixel coverage of conjunctival blood vessels (per cent coverage) determined using a conjunctival hyperemia-analysing software.
Results In omidenepag, the conjunctival hyperemia score and per cent coverage peaked at both 30 min (mean score±SD: 1.57±0.67 and 11.90%±3.66%, respectively) and then gradually decreased at 60 min (10.79%±3.32%) and 120 min (1.10±0.52) when they reached a level that was not significantly different from the baseline values. For ripasudil 0.4%, the peak time of the conjunctival hyperemia score and per cent coverage were both at 15 min (score: 2.42±0.54 and 15.26%±3.38%). The degree of conjunctival hyperemia was significantly higher for ripasudil 0.4% than that for omidenepag from 15 to 30 min in both the conjunctival hyperemia score and per cent coverage (p<0.007 by Bonferroni correction).
Conclusion Conjunctival hyperemia induced by omidenepag gradually peaks to moderate severity, though weaker compared with that induced by ripasudil 0.4%, and subsides relatively quickly.
- glaucoma
- drugs
- conjunctiva
- ocular surface
- pharmacology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Previous studies have demonstrated that conjunctival hyperemia is the most frequent complaint among patients with glaucoma and the main adverse effects of omidenepag isopropyl 0.002% were conjunctival hyperemia.
What are the new findings?
Our study demonstrated that conjunctival hyperemia induced by omidenepag gradually peaks to moderate severity, though weaker compared with that induced by ripasudil 0.4%, and subsides relatively quickly.
How might these results change the focus of research or clinical practice?
The results of this prospective clinical trial provide useful adverse information for the patients with glaucoma and clinicians about this new antiglaucoma eye-drop.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide, with approximately 60 million individuals suffering from the disease.1 Adequate reduction of intraocular pressure (IOP) to avoid visual field deterioration remains the only established treatment for the disease.2–4 Since their development 20 years ago, prostaglandin F2α analogues have become the first-line monotherapy for the treatment of glaucoma, owing to the lower rate of systemic side effects. However, prostaglandin F2α analogues are associated with specific local side effects, termed ‘prostaglandin-associated periorbitopathy,’ around the prescribed eye.5–8 These adverse effects influence the adherence of patients to the treatment of glaucoma or the results of filtering surgery.9 Omidenepag isopropyl, a selective prostaglandin E2 receptor 2 (EP2) agonist with a non-prostaglandin structure, has been developed. Studies in animals10 11 and humans12 13 have confirmed the IOP-lowering efficacy of omidenepag isopropyl. Finally, in September 2018, the omidenepag isopropyl ophthalmic solution 0.002% (EYBELIS; Santen Pharmaceutical, Osaka, Japan) was approved in Japan for the treatment of glaucoma and ocular hypertension. Different from the specific local side effects associated with prostaglandin F2α analogues, the most frequently reported adverse effects for omidenepag isopropyl 0.002% were conjunctival hyperemia (24.5%14 and 18.8%,15 followed by an increase in central corneal thickness (CCT) (11.7%),14 and macular oedema (6.4%).15 Conjunctival hyperemia is the most common cosmetic side effect occurring in patients receiving omidenepag isopropyl 0.002%. In a study investigating the most common and most uncomfortable side effects of topical antiglaucoma medications, conjunctival hyperemia was the most frequent complaint (35.6%) and the second most uncomfortable side effect (19.8%).16 One meta-analysis of conjunctival hyperemia due to prostaglandin F2α analogues showed that the utilisation of latanoprost 0.005% is associated with a lower rate of conjunctival hyperemia compared with bimatoprost 0.003%.17 Meanwhile, a novel antiglaucoma eye-drop, ripasudil hydrochloride hydrate 0.4% is a Rho-kinase inhibitor that effectively lowers IOP. The most frequently reported adverse event of this agent is also conjunctival hyperemia, occurring in more than 60% of patients.18–21 Both types of topical antiglaucoma medications induce conjunctival hyperemia. However, it is difficult to evaluate the actual extent of conjunctival hyperemia in the clinic, owing to the amount of time passed since the eye-drops were prescribed to patients. Previously, we reported the real-time course of conjunctival hyperemia induced by ripasudil 0.4% in healthy subjects22 and in patients with glaucoma.23
In previous trials of omidenepag isopropyl 0.002%,14 15 the adverse effects were judged at the clinic more than 9 hours after the last prescription. Thus, the incidence of conjunctival hyperemia in real life remains unknown. Therefore, we conducted a prospective study to investigate the detailed progression and offset of conjunctival hyperemia after a single instillation of omidenepag isopropyl 0.002% and also investigated the degree of conjunctival hyperemia compared with ripasudil 0.4%.
Materials and methods
This is a prospective, interventional, non-randomised study performed in accordance with the Declaration of Helsinki. This study involved 34 healthy subjects (22 women). The mean age of the subjects was 29.7 years (range: 23–48 years). Healthy subjects without any ocular disease and those having previously undergone ocular surgery were recruited from the hospital between November 2018 and February 2019. Written informed consent was provided by each subject prior to participation in this study.
Slit-lamp photography and measurements of IOP and CCT were performed on both eyes under fixed conditions at baseline and 15, 30, 60, 120, 180 and 360 min after a single instillation of omidenepag isopropyl 0.002% in the right eye and ripasudil 0.4% in the left eye between 8:30 and 9:00. Slit-lamp photographs were captured using an SL-D7 camera (TOPCON, Tokyo, Japan). The detailed slit-lamp photographic conditions were as follows: the angle between the slit lamp and the microscope arm was set at 30°. The camera flash light was adjusted to level 1. The slit width was set at 20 mm, and the objective magnification was set at 10×. The diffuser of the slit lamp was used. In this study, we captured two photographs for each subject: the whole bulbar conjunctiva in the front position and temporal bulbar conjunctiva in each eye. Similar to our previous reports, the photographic method was strictly fixed during this study,22 23 and the photographs were stored as JPEG images. The IOP was measured using an Icare PRO Rebound Tonometer (Tiolat Oy, Helsinki, Finland) in the seated position. The methods using the Icare PRO are established.22 24 All rebound tonometers have slightly less instrumental reliability compared with the Goldmann applanation tonometer24; however, its agreement with the Goldmann applanation tonometer is the best among the currently available rebound tonometers.24 The CCT was measured using three-dimensional corneal and anterior segment optical coherence topography (SS-1000; CASIA, Tomey, Japan). The slit-lamp photography and IOP and CCT measurements were performed by trained orthoptists (ET, YN, YF, KU, YK, SD, SO and MS) in random order.
Conjunctival hyperemia score
The whole bulbar conjunctival hyperemia at the front position was scored in each case and judged according to the Japanese guidelines for allergic conjunctival disease using whole conjunctival photography.25 In real world, the patients will notice their conjunctival hyperemia in the front position (eg, a mirror), so we used whole conjunctival photography at the front position. The grading scales were as follows: 0 (none: no hyperemia in the bulbar conjunctiva), 1 (mild: dilation of a few conjunctival blood vessels (2 or 3)), 2 (moderate: dilation of many conjunctival blood vessels (≤4 to 9 vessels)) or 3 (severe: dilation of all conjunctival blood vessels (≥10 vessels)). The grading was performed by three evaluators (ET, YN and SD)), who were blinded to one another’s findings, using the photographs captured at each of the seven time points. The mean score was used for the subsequent analysis. The degree of agreement among the three observers regarding the conjunctival hyperemia scores was also evaluated.
Pixel coverage of conjunctival vessels in the region of interest (per cent coverage)
The photographs were processed using a hyperemia analysis software program developed by the members of our group (TY and AF).25 This software was used in our previous studies, demonstrating high reproducibility of per cent coverage (r=0.998) and strong correlation to the conjunctival hyperemia score, according to the Japanese guidelines25 for allergic conjunctival disease (r=0.953).22 23 26 The software selects the optimal area of the conjunctiva and calculates the proportion of blood vessels (%) in the conjunctiva as the pixel per cent coverage. Therefore, the photographs were transferred to the software for the automatic calculation of the pixel value. For the measurement of the per cent coverage, the temporal conjunctiva (700–890 pixels width×800–920 pixels height) was assessed in all subjects because the temporal conjunctiva is the widest area among four conjunctival fields (superior, inferior, nasal and temporal). The measured location and area were constant in each individual.
Patient involvement
Patients were not directly involved in the design of this study.
Statistical analysis
The statistical analyses were performed using the statistical programming language R (V.3.1.3; R Foundation for Statistical Computing, Vienna, Austria) and Statcel 3 (OMS Publishing, Tokyo, Japan). The degree of agreement among the three observers regarding the conjunctival hyperemia scores was evaluated according to intraclass correlation coefficients (3,1). The conjunctival hyperemia score is non-parametric data as described earlier in this article, whereas per cent coverage is parametric data in normal subjects.27 Therefore, multiple comparison tests were used to investigate the differences in the conjunctival hyperemia score (Steel test) and per cent coverage (Dunnett test) over the different time points after topical administration. For comparison between groups at each time point, we used the Mann-Whitney U test for the conjunctival hyperemia score and Student’s t-test for per cent coverage (statistically significant p<0.05/7=0.007 by Bonferroni correction).
In addition, any differences in the IOP and CCT over time were evaluated through one-way analysis of variance (ANOVA) after the instillation in both eyes. For comparison between groups at each time point, we used Student’s t-test for IOP and CCT (statistically significant p<0.05/7=0.007 by Bonferroni correction). The data were expressed as the mean±SD (range). P-values <0.05 denoted statistical significance. Our sample size (n=34) can detect a 0.382 difference in the conjunctival hyperemia score among the time points, with a significance level of 5% and power of 80%, according to a SD of 0.555 in the baseline hyperemia score in the omidenepag isopropyl 0.002% eye.
Furthermore, our sample size can detect a 1.837% difference in the conjunctival hyperemia score among the time points, with a significance level of 5% and power of 80%, according to an SD of 2.665 in the baseline per cent coverage in the omidenepag isopropyl 0.002% eye.
Results
Conjunctival hyperemia score
The intraclass correlation coefficient (3,1) among the three observers for the conjunctival hyperemia score grading in all eyes was 0.391 (95% CI, 0.335 to 0.447). There are no missing data.
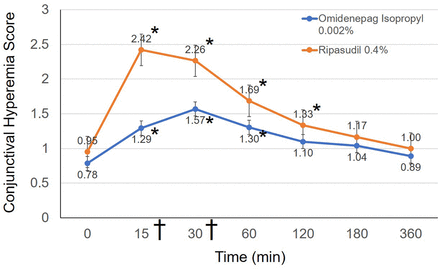
Figure 1 shows the time course for the conjunctival hyperemia score in both eyes. The conjunctival hyperemia scores at baseline and 15, 30, 60, 120, 180 and 360 min after instillation of omidenepag isopropyl 0.002% were 0.78±0.55 (0–2.33), 1.29±0.59 (0–3), 1.57±0.67 (0.33–3), 1.30±0.62 (0.33–2.66), 1.10±0.52 (0–2.33), 1.04±0.54 (0–2.33) and 0.89±0.56 (0–2.33), respectively (figure 1). The conjunctival hyperemia scores after instillation of ripasudil 0.4% were 0.95±0.45 (0.33–2), 2.42±0.54 (0.66–3), 2.26±0.51 (0.66–3), 1.69±0.48 (0.66–2.66), 1.33±0.47 (0.33–2), 1.17±0.47 (0.33–2) and 1.00±0.41 (0.33–1.66), respectively (figure 1).
Changes in the conjunctival hyperemia score. Line graphs showing the mean conjunctival hyperemia scores at each study time point in both groups. Statistically significant differences from baseline, as determined by the Steel test, are denoted by an asterisk (*). Data were presented as the means with SEs. Statistically significant difference was found between omidenepag isopropyl 0.002% and ripasudil 0.4% at 15 and 30 min (Mann-Whitney U test, statistically significant p<0.007 by Bonferroni correction). † symbol denotes statistical significance.
In eyes in which omidenepag isopropyl 0.002% was instilled, the highest score was observed at 30 min. Multiple comparison tests (Steel test) showed significant differences between the baseline score and the scores obtained at 15, 30 and 60 min (p<0.05). However, significant differences were not observed at 120, 180 and 360 min (all p>0.05). In eyes in which ripasudil 0.4% was instilled, the highest score was reported at 15 min. Multiple comparison tests (Steel test) showed significant differences between the baseline score and the scores obtained at 15, 30, 60 and 120 min (p<0.05). However, significant differences were not observed at 180 and 360 min (all p >0.05). Notably, absence of an increase in any score compared with baseline was recorded in one subject (2.8%) per group.
Instillation of ripasudil 0.4% induced significantly higher scores versus omidenepag isopropyl 0.002% at 15 and 30 min (Mann-Whitney U test, statistically significant p <0.007 by Bonferroni correction). There was no significant difference found at baseline and 60, 120, 180 and 360 min after instillation (all p >0.007).
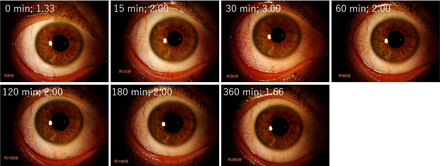
The representative time course of conjunctival hyperemia scores induced by omidenepag isopropyl 0.002% is shown in figure 2.
Representative photographs of different conjunctival hyperemia scores. The eye of a 34-year-old man, who received omidenepag isopropyl 0.002%. Figures were presented with time and conjunctival hyperemia score. The peak time was observed at 30 min (score: 3.00), and conjunctival hyperemia was gradually decreased during the measurement period.
Pixel coverage of conjunctival vessels in the region of interest (per cent coverage)
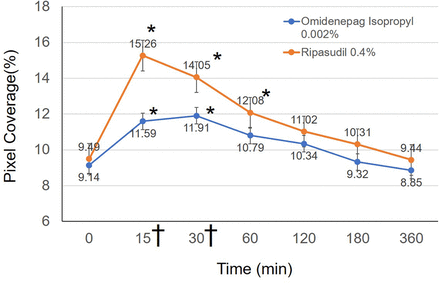
There are no missing data. The per cent coverage at baseline and 15, 30, 60, 120, 180 and 360 min after instillation of omidenepag isopropyl 0.002% were 9.28%±2.70% (4.8–16.4), 11.59%±4.09% (5.5–22.1), 11.91%±3.66% (6.5–23.4), 10.79%±3.32% (4.9–20.1), 10.34%±3.21% (5.2–20.9), 9.32%±2.87% (4.8–20.3) and 8.85%±2.60% (4.7–18.5), respectively (figure 3). The per cent coverage after instillation of ripasudil 0.4% were 9.49%±2.29% (5.4–17), 15.26%±3.38% (7.2–22.1), 14.05%±2.35% (8.5–21.1), 12.08%±3.01% (6.4–19.8), 11.02%±3.04% (5.5–18.3), 10.31%±2.46% (6.4–18.5) and 9.44%±2.03% (5.4–14.9), respectively. For omidenepag isopropyl 0.002%, the highest score was observed at 30 min, and multiple comparison tests (Dunnett test) showed significant differences between the baseline score and the scores recorded at 15 and 30 min (p <0.05). However, significant differences were not observed at 60, 120, 180 and 360 min (all p >0.05). For ripasudil 0.4%, the highest score was noted at 15 min, and multiple comparison tests (Dunnett test) showed significant differences between the baseline score and the scores at 15, 30 and 60 min (p <0.05). However, significant differences were not observed at 120, 180 and 360 min (all p >0.05). Among those who received omidenepag isopropyl 0.002%, an increase in the per cent coverage compared with baseline was not observed in three subjects (8.8%). Among those who received ripasudil 0.4%, all subjects showed an increase in the per cent coverage compared with baseline.
Changes in per cent coverage. Line graphs showing the mean per cent coverage at each study time point. Statistically significant differences from baseline, as determined by the Dunnett test, are denoted by an asterisk (*) in both groups. Data were presented as the means with SEs. Statistically significant difference was found between omidenepag isopropyl 0.002% and ripasudil 0.4% at 15 and 30 min (Student’s t-test, statistically significant p<0.007 by Bonferroni correction). † symbol denotes statistical significance.
Ripasudil 0.4% induced significantly higher scores versus omidenepag isopropyl 0.002% at 15 and 30 min (Student’s t-test, statistically significant p <0.007 by Bonferroni correction). There was no significant difference found at baseline and 60, 120, 180 and 360 min after instillation (all p >0.007).
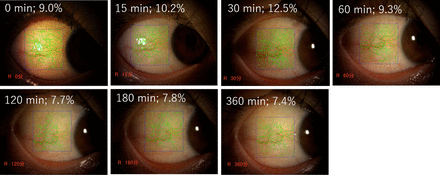
The representative time course of per cent coverage induced by omidenepag isopropyl 0.002% is shown in figure 4.
Representative photographs of different per cent coverage values. The eye of a 39-year-old woman who received omidenepag isopropyl 0.002%. The blue rectangle outlines the examined region (867 pixels (width)×907 pixels (height)). The per cent coverage of conjunctival blood vessels is shown in green. The peak time was observed at 30 min (12.5%), and conjunctival hyperemia was gradually decreased during the measurement period.
All data sets are available as online supplementary file (Online resource 1).
Supplemental material
IOP and CCT
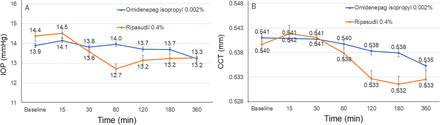
The IOP levels were not significantly changed by omidenepag isopropyl 0.002% (p=0.841 by one-way ANOVA) or ripasudil 0.4% (p=0.06 by one-way ANOVA) during the period (figure 5A). For ripasudil 0.4%, IOP showed a tendency toward decrease at 60 min (from 14.4 mm Hg at baseline to 12.7 mm Hg at 60 min). There was no significant difference found between the groups at any of the time points (all p >0.007 by Bonferroni correction).
Changes in intraocular pressure (IOP) and central cornealthickness (CCT). (A) The IOP levels were not significantly changed by omidenepag isopropyl 0.002% (p=0.841) or ripasudil 0.4% (p=0.06) during the period. Data were presented as the means with SEs. There was no significant difference found between the groups at any of the time points (p >0.007 by Bonferroni correction). (B) The CCT levels were not significantly changed by omidenepag isopropyl 0.002% (p=0.991) or ripasudil 0.4% (p=0.723) during the period. There was no significant difference found between the groups at any of the time points (p >0.007 by Bonferroni correction).
The CCT levels were not significantly changed by omidenepag isopropyl 0.002% (p=0.991 by one-way ANOVA) or ripasudil 0.4% (p=723 by one-way ANOVA) during the period (figure 5B). There was no significant difference found between the groups at any of the time points (all p>0.007 by Bonferroni correction).
Discussion
In the present study, we showed the peak time and the offset of conjunctival hyperemia induced by a novel antiglaucoma ophthalmic solution, namely, omidenepag isopropyl 0.002%. Additionally, through a comparison with ripasudil 0.4%, we can speculate the degree of conjunctival hyperemia in the real world. Omidenepag isopropyl 0.002% is a selective prostaglandin EP2, which decreases IOP by increasing outflow facility and the uveoscleral outflow.10–12 However, the apparent cause of conjunctival hyperemia remains unknown similar to that of prostaglandin F2α analogues. One potential mechanism of conjunctival hyperemia may involve stimulation of the EP2 receptor, inducing relaxation of smooth muscles. Studies have confirmed this effect as dilated retinal arterioles in rats.28 The effect of benzalkonium chloride29 or inflammation such as macular oedema (4.7%) or iritis (1.2%) which has also been reported as an adverse event15 may be another potential mechanisms of conjunctival hyperemia. Meanwhile, a probable mechanism through which ripasudil 0.4% induces conjunctival hyperemia is smooth muscle relaxation within the conjunctival blood vessel walls and modulation of vascular endothelial cells.30–32 The mechanisms of conjunctival hyperemia in each antiglaucoma eye-drop preparation are slightly different.
Our previous study showed that conjunctival hyperemia induced by ripasudil 0.4% peaks at approximately 5–15 min after administration; it tends to be moderately severe, and the symptom generally resolves within 2 hours.22 This tendency was also confirmed in the present study. Our results showed that the degree of conjunctival hyperemia induced by omidenepag isopropyl 0.002% was lower than that induced by ripasudil 0.4% and that this effect occurs early (ie, 15 and 30 min) after the instillation. Using the conjunctival hyperemia analysis software, Sumi et al reported that the per cent coverage in users of four prostaglandin analogues (ie, tafluprost, latanoprost, travoprost and bimatoprost) was 8.2%±3.9%, 11.5%±3.4%, 12.8%±5.4% and 14.5%±3.6%, respectively.33 These results were consistent with our impressions from the clinical examination. Therefore, the present results match the impressions of patients or ophthalmologists and may be useful information.
Regarding the reduction of IOP, there were no apparent changes observed in omidenepag isopropyl 0.002% instillation eyes (p=0.841 by one-way ANOVA), unlike in ripasudil 0.4% instillation eyes (p=0.06). Our previous study also showed an immediate reduction in IOP by ripasudil 0.4% (peak time: from 60 to 90 min after instillation).22 However, the previous study confirmed the reduction in IOP more than 1 week after initiating the instillation.14 15 Therefore, the immediate effect of omidenepag isopropyl 0.002% remains unknown. Additionally, in a phase III trial investigating omidenepag isopropyl 0.002%, CCT thickening was observed in 11.7% of subjects (note: not described the criteria).14 In all subjects included in the present study, there was no more than a 10 µm increase in the CCT value at each time point versus the CCT value recorded at baseline. The CCT in healthy young subjects exhibit diurnal variation of approximately 20 µm.34 Therefore, immediate changes (<10 µm) are not considered clinically important. Similar to the IOP-lowering effect of omidenepag isopropyl 0.002%, the increase in CCT may require longer time after initiating continuous instillation per day.
There are several limitations in the present study. First, the study population was small and only included healthy subjects. Therefore, the addition of omidenepag isopropyl 0.002% to the therapeutic regimen of patients already receiving another medication against glaucoma (especially another prostaglandin F2α analogue) may lead to an increased incidence of conjunctival hyperemia. Further, a larger study is required for confirming the severity of conjunctival hyperemia in both drugs. Second, the effect of long-term use on conjunctival hyperemia remains unknown. A previous study investigating conjunctival hyperemia induced by prostaglandin F2α analogues showed that it reached its peak at 15 days and started to decrease 1 month after the initiation of therapy.35 Third, the selection of the eyes to which the two drugs would be administered was not randomised in the present study. We believe that this value is nearly zero because the evaluators did not know the detailed drug information; however, there remains a possibility that this may have affected the ability of the evaluators in recording the conjunctival hyperemia score. In addition, the interobserver correlation in the conjunctival hyperemia score was relatively low (0.391) because this measurement is subjective. Therefore, an objective measurement using the conjunctival hyperemia score will be useful to support the evaluation of the conjunctival hyperemia.
Conclusion
Conjunctival hyperemia induced by omidenepag isopropyl 0.002% gradually peaks to moderate severity, though weaker compared with that induced by ripasudil 0.4%, and subsides relatively quickly. Our findings will be useful for the patient adherence in the real world.
Acknowledgments
The authors thank Enago (www.enago.jp) for English language review.
References
Footnotes
Presented at The article has been presented at the 30th Annual Meeting of the Japanese Glaucoma Society on 7 September 2019.
Contributors SN had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SN, YK, AF. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: YK, AF. Study supervision: SN, YK, AF, RA.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval This study received approval from the Institutional Review Board of Saneikai Tsukazaki Hospital (Himeji, Japan).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available in a public, open access repository.