- 1Department of Pediatrics, University of Calgary Cumming School of Medicine, Calgary, Canada
- 2University of Toronto at Scarborough, Toronto, Canada
- 3Department of Obstetrics and Gynecology, University of Calgary Cumming School of Medicine, Calgary, Canada
- 4Department of Ophthalmology, University of Calgary Cumming School of Medicine, Calgary, Canada
- Correspondence to Dr Kamran Yusuf; kyusuf{at}ucalgary.ca
- Received 6 October 2016
- Revised 31 January 2017
- Accepted 9 March 2017
Abstract
Objective To evaluate the relationship between pre-eclampsia and development of retinopathy of prematurity (ROP) in infants with birth weight of <1500 g and/or gestation <31 weeks.
Methods A retrospective cohort study comprising infants born to mothers with pre-eclampsia between January 2007 and June 2010 at a single tertiary care centre. Their ROP outcome was compared with infants born to the next two normotensive mothers with a ±1 week gestational age difference. Pearson χ2 test was used for categorical variables and Mann-Whitney U test was used for continuous variables. Multivariable regression was used to estimate the OR of ROP with prenatal pre-eclampsia exposure and adjust for confounders.
Results Of the 97 infants in the pre-eclampsia group, 27 (27%) developed ROP and of the 185 infants in the normotensive group, 50 (27%) developed ROP. On multivariable regression modelling, pre-eclampsia was not a risk factor for the development of ROP (OR 1.4, 95% CI 0.46 to 4.1). Gestational age, intrauterine growth restriction and blood transfusion were significant risk factors for the development of ROP.
Conclusions In our cohort, pre-eclampsia was not a significant risk factor for the development of ROP. Intrauterine growth restricted infants of pre-eclamptic and normotensive mothers were at higher risk of ROP.
- Retina
- Neovascularisation
- Epidemiology
- Angiogenesis
Key messages
Studies on pre-eclampsia and retinopathy of prematurity (ROP) show variable results. Some are from earlier periods when nutritional practices, mechanical ventilation, supplemental oxygen and antenatal corticosteroid use were different from current standard of care. Some studies have included cases of maternal hypertension other than pre-eclampsia in their cohorts, there is marked variation in the gestational ages and some have substantial missing data.
In a well-defined cohort from a recent era, we demonstrate pre-eclampsia is not associated with ROP, either as a protective or as risk factor. There were no missing data and high level of antenatal corticosteroid use. Unlike previous studies, chorioamnionitis was defined pathologically, and oxygen saturation targets and mechanical ventilation strategies were consistent during this period.
This is a retrospective cohort study from a single centre potentially subject to confounding.
Prematurity and intrauterine growth restriction, whether in pre-eclamptic or normotensive pregnancies, are major risk factors for the development of ROP.
Introduction
Retinopathy of prematurity (ROP), a vasoproliferative disorder of the developing retina, is one of the more severe consequences of preterm birth and a major cause of childhood blindness and visual impairment in the developing and developed world.1 The disease is more common in infants less than 31 weeks’ gestation with infants of lesser gestation at higher risk and severity of ROP. In infants with a birth weight of <1500 g, the reported incidence of ROP ranges from 20% to 50% in different populations.1–3
Described first as ‘retrolental fibroplasia’ in 1942, the pathogenesis of ROP is still not fully understood but involves an intricate interplay between retinal blood vessels, oxygen, angiogenic and growth factors, of which vascular endothelial growth factor (VEGF) is the most important.4 ROP is described in two phases. The first phase begins after preterm birth secondary to the hyperoxic extrauterine environment and involves suppression of VEGF, vaso-obliteration with cessation of retinal blood vessel growth and endothelial apoptosis. The second phase is proliferative and involves neovascularisation of retinal vasculature by vasoactive factors such as VEGF, produced secondary to a hypoxic and avascular retina of phase 1. The second phase begins around 32 weeks postmenstrual age but can have a wide range of onset.2 4 Prematurity continues to be the single most important risk factor for ROP, but given its developmental nature, efforts have recently focused on identifying antenatal risk factors for ROP.
Pre-eclampsia is characterised by new-onset maternal hypertension and proteinuria at or after 20 weeks of gestation. Although the aetiology of pre-eclampsia remains unknown, evidence suggests that a hypoxic and dysfunctional placenta produces excess antiangiogenic factors, soluble fms-like tyrosine kinase 1 and soluble endoglin, resulting in decreased maternal levels of angiogenic factors, VEGF and placental growth factor. This imbalance between angiogenic and antiangiogenic factors is responsible for maternal endothelial damage and manifestations of pre-eclampsia.5 How this antiangiogenic intrauterine milieu in pre-eclampsia affects retinal vasculature development is not well understood. Given that angiogenic factors such as VEGF are involved in the pathogenesis of ROP, and pre-eclampsia is associated with lower levels of VEGF, we hypothesised that preterm infant less than 31 weeks’ gestation and\or with a birth weight of <1500 g born to mothers with pre-eclampsia would be at a lower risk of developing ROP when compared with infants born to normotensive mothers.
Methods
The Neonatal Intensive Care Unit (NICU) in Calgary maintains a prospectively collected electronic database of all infants admitted to the NICU. Infants less than 31 weeks gestational age (GA) and/or with a birth weight of <1500 g born to mothers with pre-eclampsia between January 2007 and June 2010 were included in the study. Their outcome was compared with infants born to the next two normotensive mothers with a ±1 week GA difference. In case of missing data, the infant’s medical record charts were reviewed. The Conjoint Health Research Ethics Board of the University of Calgary approved the study.
Maternal data
Pre-eclampsia was defined as systolic blood pressure ≥140 mm Hg or a diastolic level of (Korotkoff 5) ≥90 mm Hg on two or more occasions at least 4–6 hours (but not more than 7 days) apart after 20 weeks’ gestation in a woman with previously normal blood pressure. Proteinuria was defined as ≥0.3 g protein in a 24-hour urine sample. When a 24-hour urine sample was not feasible, ≥0.3 g/L protein or ≥1+ on a dipstick test strip on two random urine samples taken at least 4–6 hours apart was used as criteria for proteinuria.6Histological chorioamnionitis was defined as infiltration of polymorphonuclear leucocytes in the fetal membranes and chorionic plate.7 Antenatal steroids were considered a course if more than 12 hours had elapsed after the first dose.
Neonatal data
Screening for ROP in Calgary is based on published recommendations and includes infants <1500 g and/or gestation <31 weeks as well as infants with birth weight of >1500 g and gestation >30 weeks who have a severe and complicated clinical course. All infants have their first examination at 28 days of life and then at 1–2 weeks interval depending on the findings of the initial examination. All funduscopic examinations were performed by two paediatric ophthalmologists.
The severity of ROP was based on the International Classification of ROP.8 No ROP was designated as stage 0, stage 1 was a line of demarcation, stage 2 a ridge, stage 3 intravitreous neovascularisation, stage 4 partial retinal detachment and stage 5 total retinal detachment. Severe ROP was defined as >stage 2. Plus disease, an indication of severity of ROP, was defined as venous dilation and arteriolar tortuosity of the posterior retinal vessels sometimes including vascular engorgement of the iris, poor papillary dilation and vitreous haze.
GA was assessed in the following order of preference: date of embryo transfer for in vitro fertilisation, first trimester fetal ultrasound, second trimester fetal ultrasound, date of the last menstrual period and postnatal assessment using the modified Ballard score.
Intraventricular haemorrhage (IVH) was graded on Papile’s criteria.9 The diagnosis of necrotising enterocolitis (NEC) was based on modified Bell’s criteria.9 Neonatal sepsis was the presence of single organism from either blood or cerebrospinal fluid culture. Respiratory distress syndrome (RDS) was diagnosed based on the presence of signs of respiratory distress, a typical chest X-ray and/or need for surfactant. Patent ductus arteriosus (PDA) was diagnosed based on clinical signs and echocardiography. Bronchopulmonary dysplasia (BPD) was defined as the need for supplemental oxygen or any form of ventilation including continuous positive airways pressure at 36 weeks postmenstrual age.9 Intrauterine growth restriction (IUGR) was defined as birth weight less than the 10th percentile based on the growth charts of Kramer et al.10
Exclusion criteria included infants born with any congenital malformations or chromosomal anomalies, mothers with chronic hypertension, maternal renal, cardiovascular or autoimmune disease, substance abuse, TORCH infections and infants who died before their first eye examination.
As the distribution of the relevant variables was not normal, we chose conservative non-parametric analysis for continuous variables, using the Mann-Whitney U test. Categorical variables were compared using the χ2 or Fisher’s exact test as appropriate. To identify risk factors for the development of ROP, multivariable logistic regression with backward elimination approach was performed. Any risk factors that demonstrated associations, whether statistically significant or judged to be clinically significant, with both pre-eclampsia and ROP but were not intermediate variables, were included in the modelling process as possible confounders.11 The least significant variables were then removed until all remaining variables were significant at p value of 0.2. The p value of 0.2 was set conservatively as an entry for variables to proceed to the next step in the analysis.12The adjusted OR values and their 95% CI are reported. A p <0.05 was considered significant. Data were analysed using STATA V.13.
Results
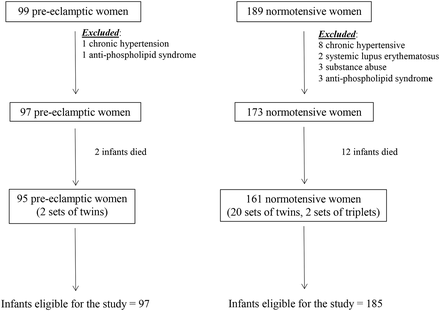
During the study period, there were 99 women with pre-eclampsia and 189 women in the normotensive group who delivered infants less than 31 weeks' gestation and/or with a birth weight of <1500 g. Figure 1 shows the mothers and infants who were excluded resulting in 95 women and 97 infants in the pre-eclampsia group and 161 women and 185 infants in the normotensive group.
Flow diagram of patients included in the study.
There was no difference in the maternal age or gravidity between the two groups. Although the number of primigravidas and antenatal steroid use was higher in the pre-eclampsia group, the difference was not statistically significant. Smoking rates, number of Caucasians and diabetes were similar between the two groups. Caesarean section rates were significantly higher and pathological chorioamnionitis and multiple births were lower in the pre-eclampsia group (table 1).
Maternal characteristics
Although the GA was significantly lower in the normotensive group, the birth weights were higher. IUGR rates were significantly higher in the pre-eclampsia group. There was no difference in SNAP-PE (Score for Neonatal Acute Physiology-Physiological Extension) scores, number of males, RDS, PDA, IVH, NEC, BPD, blood transfusion rates, duration of oxygen use and mechanical ventilation between the two groups. Sepsis rates were higher in the normotensive group. Importantly, there was no difference in ROP, including severe ROP (>stage 2) between the two groups (table 2).
Neonatal characteristics and outcomes
Out of the total cohort of 282 infants, 76 (27%) developed ROP.
Bivariate analysis confirmed a number of known risk factors for ROP and included GA, birth weight, SNAP-PE scores, oxygen and mechanical ventilation days, IUGR, RDS, surfactant use, PDA, IVH, NEC and BPD (table 3). There was no difference in the mode of delivery, multiple birth, chorioamnionitis and gender between the two groups. Out of 76 infants with ROP, 26 (34%) were delivered to mothers with pre-eclampsia and out of the 206 infants without ROP, 71 (34%) were born to mothers with pre-eclampsia. This was statistically non-significant, p=0.54.
Characteristics of infants with and without ROP
On logistic regression including significant factors, pre-eclampsia was neither protective nor a risk factor for the development of ROP (OR 1.7, 95% CI 0.6 to 4.7, p=0.29). Lower GA, blood transfusion and IUGR were risk factors for the development of ROP (table 4).
Results of multivariate regression modelling risk of ROP
Analysis of data for infants ≤28 weeks’ gestation and <1000 g birth weight did not change the results.
Discussion
The results of our study demonstrate that pre-eclampsia is not associated with the development of ROP, either as a protective or risk factor. The results also confirmed a number of known risk factors of ROP, including GA, IUGR and blood transfusions.13 14 Our results are similar to the report by Huang et al14 who, using a large cohort of very low birthweight infants, demonstrated pre-eclampsia to be not associated with the development of ROP but found IUGR and blood transfusions to be significant risk factors. As in our study, gender, RDS, days of oxygen supplementation and mechanical ventilation were not risk factors for ROP. Other studies have also reported no association of pre-eclampsia with ROP.15–18 More recently, a meta-analysis on the association of ROP with gestational hypertensive disorders, which included studies on gestational hypertension and pre-eclampsia, demonstrated no association.19
Reports of the association of pre-eclampsia and ROP are, however, conflicting with pre-eclampsia being reported protective and also as a risk factor for the development of ROP.20–31 Investigators reporting pre-eclampsia as protective for ROP have attributed it to the lower VEGF levels and higher levels of antiangiogenic factors in pre-eclampsia.20 While reports of maternal levels of angiogenic and antiangiogenic factors in pre-eclampsia are relatively consistent, reports of umbilical cord blood and neonatal levels are equivocal at best, with reported levels being higher, lower and the same as infants of normotensive mothers.32 33 It has also been speculated that amniotic fluid antiangiogenic factors in pre-eclampsia could access the retina through the corneal epithelium and in this way protect against ROP.20 21 The few reports of angiogenic and antiangiogenic factors in amniotic fluid in pre-eclamptic and normotensive pregnancies also show disparate results.33 34 It is also important to note that the pathophysiology of ROP is complex, and an antiangiogenic state with low levels of VEGF would be of benefit in the second phase of ROP, remote and several weeks away from the time of birth and the antiangiogenic intrauterine milieu of pre-eclampsia.4 Some authors have also suggested that intrauterine stress associated with pre-eclampsia could lead to the accelerated development of retinal blood vessels, lessening the chances of ROP.22 However, IUGR, which is also associated with intrauterine stress, is a risk factor rather than protective for the development of ROP in most if not all studies on risk factors of ROP. Although angiogenic factors such as VEGF are important for retinal vasculature development, the role these factors play in fetal retinal development in pre-eclampsia remains unclear.
An equal number of investigators have reported pre-eclampsia to be a risk factor for the development of ROP.25–29 Although the exact mechanism of how pre-eclampsia leads to ROP is not established, various reasons have been reported. Increased oxidative stress and inflammation associated with pre-eclampsia have been suggested to cause impaired retinal vascularisation resulting in ROP.25 35 However, in a recent systematic review and meta-analysis, Mitra et al36 reported chorioamnionitis was not associated with ROP. Lee et al29 using the Extremely Low Gestational Age Newborns study cohort comprising 1199 infants, reported prelabor premature rupture of membranes and placental abruption, both conditions associated with inflammation, to have a reduced risk of ROP.29 Low levels of insulin-like growth factor (IGF)-1 in pre-eclampsia have also been implicated in the increased risk of ROP.37 As with angiogenic and antiangiogenic factors, reports of reduced maternal levels of IGF-1 are consistent but reports of umbilical cord blood levels are conflicting with reports of higher umbilical cord levels of IGF-1 in pre-eclampsia.38 39
There are several reasons for the conflicting results on the association between pre-eclampsia and ROP. These include different definitions of pre-eclampsia and ROP, inclusion of cases of hypertension other than pre-eclampsia, different GAs, different time of eye examinations, varying rates of antenatal corticosteroid use and performing only univariate analysis rather than multiple regression. Some investigators have excluded infants who died before their first eye examination and some have not. Studies that have investigated risk factors for ROP and not pre-eclampsia specifically, generally have small numbers of infants with pre-eclamptic mothers.21 23 24 26 Importantly, some studies are from an earlier time period when oxygen use, ventilator and nutritional practices were different from current practice.23 27
The incidence of all ROP and severe ROP in our cohort was 27% and 6.7%, respectively, not different from that reported by the Canadian Neonatal Network (www.canadianneonatalnetwork.org). On multiple logistic regression, we did not find oxygen and mechanical ventilation to be risk factors for ROP. This may be because the NICU in Calgary has strict targets for oxygen saturations maintaining them between 85% and 95%.40 In addition, the emphasis is on non-invasive ventilation with early extubation of intubated infants. Mechanical ventilation may also be a confounding variable to oxygen supplementation. A recent study from Denmark with a large cohort also did not find oxygen duration to be a risk factor for ROP.13
The strengths of our study include a well-defined cohort from a recent era during which there was no change in clinical practice with high rates of antenatal corticosteroids use, and the ophthalmologists performing the eye examination were not aware of the diagnosis of pre-eclampsia. Another strength is the histopathological basis of chorioamnionitis in our study as correlation with clinical chorioamnionitis is poor. There were no missing clinical data or missed eye examinations. We also used GA to define our cohort, thereby minimising the confounding due to IUGR. Limitation of the study is that it is a single-centre study, making generalisations difficult. However, multicentre studies, although having large sample sizes, are beset with variation in clinical practice, differing rates of ROP between centres and substantial relevant data may also be missing.15 27
In summary, contrary to our hypothesis, we did not find pre-eclampsia to be associated with ROP, either as a protective or risk factor. Although there may be several reasons, the literature on pre-eclampsia and ROP is conflicting and inconsistent, with studies reporting increased risk, protective effect and no effect. The study confirmed known risk factors for ROP, including prematurity, blood transfusions and IUGR status.
Footnotes
Acknowledgement The authors gratefully acknowledge the funding provided by the Alberta Children’s Hospital Foundation and Alberta Children’s Hospital Research Institute for this study.
Contributors KY made substantial contributions to the conception, design and acquisition of data, analysis and interpretation. He was also involved in drafting and revising the manuscript. BA contributed to the conception and design, wrote the first draft and also helped with data analysis and interpretation and revision of the manuscript. OS helped in design, acquisition of data and writing of the manuscript. NS was involved in the conception and design, acquisition of data and writing and critiquing of the manuscript. AE was involved in conception and design and providing critical appraisal of the manuscript.
Competing interests None declared.
Patient consent Retrospective cohort study.
Ethics approval Conjoint Health Research Ethics Board, University of Calgary.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.