Abstract
Interleukin-1β (IL-1β) is a crucial mediator in the pathogenesis of inflammatory diseases at the periphery and in the central nervous system (CNS). Produced as an unprocessed and inactive pro-form which accumulates intracellularly, release of the processed cytokine is strongly promoted by ATP acting at the purinergic P2X7 receptor (P2X7R) in cells primed with lipopolysaccharide (LPS), a Toll-like receptor (TLR) 4 ligand. Microglia are central to the inflammatory process and a major source of IL-1β when activated. Here we show that purified (>99%) microglia cultured from rat cortex, spinal cord and cerebellum respond robustly to ATP-dependent IL-1β release, upon priming with a number of TLR isoform ligands (zymosan and Pam3CSK4 for TLR2, poly(I:C) for TLR3). Cytokine release was prevented by a P2X7R antagonist and inhibitors of stress-activated protein kinases. Enriched astrocytes (≤5% microglia) from these CNS regions displayed responses qualitatively similar to microglia but became unresponsive upon eradication of residual microglia with the lysosomotropic agent Leu-Leu-OMe. Activation of multiple TLR isoforms in nervous system pathology, coupled with elevated extracellular ATP levels and subsequent P2X7R activation may represent an important route for microglia-derived IL-1β. This phenomenon may have important consequences for neuroinflammation and its position to the common pathology of CNS diseases.
Similar content being viewed by others
Introduction
Inflammation is fundamentally a protective cellular response aimed at removing injurious stimuli and initiating the healing process. However, prolonged, inflammation overrides the bounds of physiological control and eventually becomes destructive1. Inflammation is increasingly seen as a key feature in the pathobiology of chronic pain, neurodegenerative diseases, stroke, spinal cord injury and perhaps even neuropsychiatric illness2,3,4,5. Further, chronic stress may induce transient spinal neuroinflammation, triggering long-lasting anxiety-induced hyperalgesia6. The reported ability of inflammation-activated signalling pathways in the brain's hypothalamus to control the production of ageing related hormones provides a link between inflammation, stress responses and ageing7. Inflammation thus constitutes an important target for neuronal protection in neurodegenerative disorders and neuropathic pain, the latter resulting from damage or disease affecting the somatosensory system8.
An extensive communication exists between the immune system and the central nervous system (CNS), with inflammatory cytokines occupying a key niche in this network, regulating host responses to infection, inflammation, stress and trauma. Microglia, the brain's principal resident immune cell population are central to the inflammatory response and can be activated by distress signals released from neighbouring cells9. Microglia that enter a pro-inflammatory state are largely activated by pattern recognition receptors (PRRs), fundamental components of the host innate immunity. PRRs identify pathogen-associated molecular patterns (PAMPs), which are linked with microbial pathogens or cell stress, as well as danger-associated molecular patterns released during cell damage. Toll-like receptors (TLRs) are a subfamily of PRRs abundantly expressed in a number of CNS cell types and, in particular, microglia. To date, 10 functional TLRs have been identified in humans and as many as 12 in mouse10. Each TLR recognizes a specific array of PAMPs. For example, TLR3 specifically engages double-stranded RNA, indicating a role in host defence against viruses11, whereas TLR2 shows affinity for a wide range of PAMPs originating from bacteria, virues, fungi and parasites12. TLR4 is triggered not only by lipopolysaccharides (LPS) from Gram-negative bacteria, but also recognizes danger-associated molecular patterns released by injured tissue13,14. Recruitment of TLRs contributes to inflammation by amplifying pro-inflammatory mediator release15,16. In the case of TLR4, a major route of such amplification may be via the purinergic receptor P2X7 (P2X7R). Interleukin-1β (IL-1β) is now viewed as master regulator of neuroinflammation17; it makes an important contribution to cellular activation and cytokine production. As such, IL-1β plays a crucial role in the pathogenesis of acute and chronic diseases of both the peripheral nervous system and CNS18,19,20.
Although LPS is a potent stimulus for IL-1β synthesis, endotoxin-dependent release of the cytokine from microglia is a rather inefficient process with most of the secreted IL-1β in the unprocessed and inactive pro-form21. However, ATP, via activation of P2X7R and caspase 1 within the context of the inflammasome is a powerful stimulus for the secretion of active mature IL-1β. This activation appears to operate as a two-step process: for example, TLR ligands like LPS prime cells (signal 1) by inducing the transcription and translation of IL-1β, followed by a secondary signal such as ATP (signal 2) to trigger formation of the inflammasome complex that leads to caspase 1 activation and cleavage/release of IL-1β22,23. P2X7R-triggered IL-1β maturation and export may thus represent a major contributor to this cytokine pool22,24. However, it is not known whether or not this process occurs also across CNS regions and TLR family members. This is an important point, given the reported heterogeneity in activation responses in microglia from different CNS regions25. The present study was therefore designed to examine the ability of ATP to promote IL-1β release from cortical, spinal cord and cerebellar microglia activated with TLR2, TLR3 and TLR4 agonists, in a P2X7R-dependent manner. Because astrocytes may influence the sensitivity of microglia to a priming stimulus26, these experiments were carried out using also both enriched and nominally microglia-free astrocytes.
Results
Microglia from cortex, spinal cord and cerebellum release IL-1β in response to TLR agonists in an ATP-dependent manner
In the brain, IL-1β is mainly produced by activated microglia27. Although bacterial endotoxin is a potent activator of IL-1β transcription and translation, release of the cytokine is a rather inefficient process21. ATP, by activating P2X7R is a powerful stimulus for secretion of mature IL-1β from microglial cell lines which have undergone a short priming with LPS22,27,28. This process occurs in primary cortical microglia, as well26 (Fig. 1a). While not described previously, primary cultures of rat cerebellar microglia (Fig. 1a) and rat spinal cord (Supplementary Fig. S1a) also release IL-1β in a LPS- and ATP-dependent fashion, thus indicating that cortex does not represent a special case.
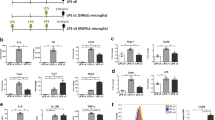
Extracellular ATP causes release of IL-1β from purified rat cortical and cerebellar microglia primed with TLR2, TLR3 and TLR4 ligands.
Cortical (solid bars) or cerebellar (hashed bars) microglia cultured in 96 well plates were pretreated for 30 min with 10 μM SB202190 (‘SB’), 10 μM SP600125 (‘SP’) or 10 μM A740003, followed by a 2-h incubation with: (a) 1 μg/ml LPS for TLR4; (b) 10 μg/ml zymosan (‘Zym’) or (c) 0.3 μg/ml Pam3CSK4 (‘Pam’) for TLR2; (d) 50 μg/ml poly(I:C) (‘P’) for TLR3. ATP was then added to a final concentration of 5 mM. After a further 60 min incubation culture medium was collected for IL-1β analysis by ELISA. Note the different scales used on the y-axes; poly(I:C) appeared to be the least efficacious of the TLR ligands tested. Each indicated treatment group applies to both cell populations. Data are means ± s.e.m. (n = 3). ***p < 0.001 and °°°p < 0.001 vs TLR activator/ATP group for cortical and cerebellar microglia, respectively. Similar results were obtained in two other experiments.
P2X7R-triggered IL-1β maturation and export may contribute significantly to this cytokine pool in nervous system pathologies22,24. An important question is thus whether or not other TLR ligands are capable of functioning as priming agents. Microglia express functional TLR229 and TLR330 in addition to TLR4. TLR signaling pathways may be involved in neurodegenerative disorders31, including motor neuron disease32, cerebral hypoxia-ischemia15,16,33 and blood-spinal cord barrier dysfunction after ischemia/reperfusion injury34, along with microglial TLR229 and TLR330 in preclinical pain models. For these experiments zymosan and Pam3CSK4 (TLR2 activators)35 and poly(I:C), an activator of TLR336 were used. None of the TLR ligands tested nor ATP treatment alone caused appreciable amounts of IL-1β to accumulate in the culture medium, whilst pretreatment with zymosan, Pam3CSK4 and poly(I:C) followed by ATP challenge resulted in a marked release of IL-1β from cortical (Fig. 1b–d), cerebellar (Fig. 1b–d) and spinal cord (Supplementary Fig. S1b–d) microglia. The ATP-dependent release of IL-1β in all cases was blocked by the potent and selective P2X7R antagonist A74000337 (Fig. 1, Supplementary Fig. S1).
Stress-activated protein kinase signalling and TLR/ATP-dependent IL-1β release from CNS microglia
The signalling pathway(s) involved in TLR/ATP-dependent IL-1β release from primary CNS microglia was explored using inhibitors for the stress-responsive protein kinases p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK). The p38 inhibitor SB202190 and the JNK inhibitor SP600125 (10 μM each) produced a significant reduction in IL-1β accumulation in the culture medium, independent of microglial cell population and TLR activator used, suggesting a common mechanism of action (Fig. 1 and Supplementary Fig. S1). In contrast, the MAPK kinase inhibitor PD98059 was ineffective (data not shown). Neither SB202190 nor SP600125 prevented the reduction in intracellular IL-1β content resulting from incubation of microglia with TLR ligand followed by ATP (see Fig. 2 for examples), indicating that blocking p38 or JNK does not interfere with P2X7R action. This is in contrast to the action of the P2X7R antagonist A740003, which effectively restored the intracellular pool of IL-1β in TLR ligand/ATP-treated spinal cord (Fig. 2a) and cerebellar (Fig. 2b) microglia – most likely a consequence of blocking P2X7R-dependent cleavage of IL-1β precursor to the releasable mature form. However, effects of SB202190 and SP600125 on IL-1β levels in the culture medium can probably be attributed to their inhibition of IL-1β production intracellularly: in cortical microglia incubated 3 h with zymosan ( = 100%), SB202190 and SP600125 reduced IL-1β content to 18.8 ± 0.3% and 53.7 ± 14.5%, respectively, while the value for zymosan + A740003 was 86.6 ± 8.2% (mean ± s.e.m., n = 3). Using LPS instead, the corresponding intracellular IL-1β values were 34.6 ± 8.7%, 30.0 ± 3.2% and 86.5 ± 9.3%, respectively, for SB202190, SP600125 and A740003.
A740003, but not SB202190 or SP600125 prevents the ATP-dependent fall in intracellular IL-1β in TLR agonist-primed rat spinal cord and cerebellar microglia.
Spinal cord (a) and cerebellar (b) microglia cultured in 96 well plates were pretreated for 30 min with 10 μM SB202190 (‘SB’), 10 μM SP600125 (‘SP’) or 10 μM A740003 (‘A74’), followed by a 2-h incubation with 10 μg/ml zymosan (a) or 1 μg/ml LPS (b). ATP was then added to a final concentration of 5 mM. After a further 60 min cell lysates were prepared for IL-1β analysis by ELISA. Data are means ± s.e.m. (n = 3). **p < 0.01 vs zymosan + ATP; ***p < 0.001 vs LPS + ATP.
Microglia, but not astrocytes produce IL-1β in response to TLR agonists
Astrocyte cultures frequently contain minor numbers of contaminating microglia, which can complicate interpretation of responses38 and modify astrocyte responses in some cases39. Further, pro-inflammatory stimuli can affect the expression of similar genes in microglia and astrocytes38,40. The lysosomotropic agent L-leucyl-L-leucine methyl ester (Leu-Leu-OMe)41 was employed to eradicate residual microglia from the monolayers of enriched astrocytes26,42,43,44. Under the conditions used, Leu-Leu-OMe does not affect astrocyte vitality26,42. In Leu-Leu-OMe-treated cultures of cortical, spinal cord and cerebellar astrocytes, LPS priming (2 h) followed by a 1-h exposure to ATP failed to elicit release of IL-1β into the medium – in contrast to the behaviour of parallel astrocyte cultures not treated with Leu-Leu-OMe (Fig. 3a). Analogous results were obtained when the various astrocyte populations were first primed with zymosan (Fig. 3b), Pam3CSK4 (Fig. 3c) or poly(I:C) (data not shown) for 2 h and then exposed to ATP for 1 h. Treatment with Leu-Leu-OMe has been shown efficacious in removing microglia from cortical and spinal cord enriched astrocytes26. Real-time polymerase chain reaction (RT-PCR) analysis of Leu-Leu-OMe-treated cerebellar astrocytes showed Iba1 gene expression to be 0.81 ± 0.16% (n = 3) of non-treated cells, indicating eradication of this contaminating cell population. Lack of IL-1β release from Leu-Leu-OMe-treated enriched astrocytes was not likely a consequence of interference with the release process. The intracellular accumulation of IL-1β in LPS-only stimulated cultures of enriched astrocytes was also abolished by Leu-Leu-OMe treatment (Fig. 4b), suggesting the removal of an agonist-responsive cell population. The difference in total IL-1β content between the LPS and LPS/ATP groups did not differ significantly (Fig. 4c), indicating that the difference in intracellular IL-1β content between these two groups (Fig. 4b) could be accounted for by release of the cytokine into the culture medium (Fig. 4a).
L-leucyl-L-leucine methyl ester abolishes IL-1β release triggered by LPS, zymosan and Pam3CSK4 plus ATP from enriched cortical, spinal cord and cerebellar astrocytes.
Enriched cortical (solid bars), spinal cord (shaded bars) and cerebellar (hatched bars) astrocytes cultured in a 96 well plate were treated with 50 mM L-leucyl-L-leucine methyl ester (L-LME) for 60 min and returned to fresh culture medium for 24 h. The cultures were then incubated in serum-free medium for 2 h with: (a) 1 μg/ml LPS, (b) 10 μg/ml zymosan, or (c) 0.3 μg/ml Pam3CSK4, each followed by addition of 5 mM ATP. After a further 60 min incubation culture medium was collected for IL-1β analysis. Data are means ± s.e.m. (n = 3). Arrows indicate that a given treatment applies to all three cell populations. In some cases no bar is visible, as the IL-1β was below the limit of detection.
Extracellular and intracellular IL-1β content derived from enriched cortical astrocytes incubated with LPS ± ATP.
Enriched cortical astrocytes cultured in a 96 well plate were incubated in serum-free medium with 1 μg/ml LPS for 2 h followed by addition of 5 mM ATP or not. After a further 60 min incubation culture medium (a) and cell lysates (b) were collected for IL-1β analysis. In some cases cells and prior to LPS/ATP challenge, cells were treated the previous day with 50 mM Leu-Leu-OMe (L-LME) for 60 min and returned to fresh culture medium for 24 h. Panel (c) presents the sum values for intracellular + extracellular IL-1β. Total IL-1β content for LPS and LPS/ATP did not differ significantly, indicating that the reduced intracellular content for LPS/ATP (panel B) could be accounted for by that released into the culture medium (panel A). Production of the ATP-dependent component of IL-1β was prevented by inclusion of the selective P2X7R antagonist A-740003 (not shown). Data are means ± s.e.m. (n = 3).
Previous studies showed that cortical and spinal cord microglia responded to LPS challenge far more robustly in the presence of astrocytes26. Comparison of Figure 2a (purified cerebellar microglia) with Figure 3b (enriched cerebellar astrocytes) shows IL-1β release from the latter (containing ≤ 5% microglia) which appears to be disproportionate for the numbers of microglia present. To test this, Leu-Leu-OMe-treated cerebellar astrocytes were cultured together with the addition of increasing numbers of cerebellar microglia. Under these conditions the co-cultures displayed a heightened priming response, whereby LPS sensitized the cells to ATP-induced release of IL-1β (Fig. 5, upper panel). Equivalent numbers of microglia alone, however, were essentially devoid of a priming response and IL-1β release (Fig. 5, lower panel).
Cerebellar microglia display a heightened LPS sensitization to ATP-induced release of IL-1β in the presence of Leu-Leu-OMe-treated cerebellar astrocytes.
Upper panel: Enriched cerebellar astrocytes (50 × 103) cultured in a 96 well plate were treated with 50 mM Leu-Leu-OMe (5th - 12th columns) for 60 min and returned to fresh culture medium for 24 h. After this time the indicated numbers of purified cerebellar microglia were added to the microglia-depleted astrocyte cultures and incubation continued for a further 24 h. The cultures were then incubated in serum-free medium with 1 μg/ml LPS for 2 h followed by addition of ATP to a final concentration of 5 mM. After a further 60 min incubation culture medium was collected for IL-1β analysis. Lower panel: The same numbers of microglia were cultured in a parallel plate, treated with LPS and ATP as above and medium analyzed for IL-1β content. Data are means ± s.e.m. (n = 3).
Earlier studies pointed to a lack of soluble astrocyte-derived factors as being responsible for imparting LPS sensitivity to microglia in terms of mediator release26, suggesting instead a role for physical contact between these two cell populations. This question was examined further using a two-chamber cell culture system, in which an upper layer (insert) of microglia is separated from a lower layer of astrocytes by means of a porous membrane that allows for communication between the compartments. A 24-h LPS incubation of astrocytes only resulted in a very small quantity of IL-1β release (Table 1, upper box) but much greater intracellular accumulation, which was reduced by ≥90% following L-LME treatment (Table 1, lower box). LPS addition to the microglia compartment also produced a small release of IL-1β but far more intracellularly; interestingly, the presence of LPS in the lower chamber also resulted in IL-1β release by microglia (most likely a result of trans-chamber LPS passage). LPS-treated microglia did not influence IL-1β expression by L-LME-treated astrocytes, either extra- or intracellularly. Although the intracellular content of IL-1β in microglia was greater in the presence of LPS- (and L-LME)-treated astrocytes compared to direct LPS treatment of the microglia (2218 ± 143 and 1407 ± 63 pg, respectively) values for IL-1β release in both cases were similar. Moreover, immunocytochemical analysis of TLR ligand-treated enriched cortical astrocyte cultures showed a lack of coincidence between glial fibrillary acidic protein (GFAP)-positive cells (astrocytes) and IL-1β-immunoreactive cells (Supplementary Fig. S2).
Discussion
Microglia, the brain's resident mononuclear phagocytic cells45 are critical for innate and adaptive responses within the CNS. These immune cells recognize and are activated by various PAMPs and play a pivotal role in neuroinflammatory processes by releasing a plethora of pro-inflammatory mediators, including IL-1β. As a frontline player in the pathogenesis of acute and chronic inflammatory diseases in the nervous system19, IL-1β stimulates the production of other cytokines, such as IL-6 and IL-8, as well as inducible nitric oxide synthase, chemotactic factors and adhesion molecules, thereby attracting peripheral mononuclear cells to cross the blood-brain barrier46. Here, we show that ATP-dependent release of IL-1β occurs in microglia (but not astrocytes) cultured from several CNS regions following a priming stimulus with a number of PAMPs, as exemplified by ligands of TLR2, TLR3 and TLR4. The generality of this phenomenon may have important consequences for neuroinflammation and its position to the common pathology of several acute and chronic CNS diseases.
It has been proposed that in multiple chronic disease states and in ageing, microglia are primed by prior pathology, or by genetic predisposition, to respond more vigorously to subsequent inflammatory stimulation, thus transforming an adaptive CNS inflammatory response to systemic inflammation with deleterious consequences47. Within this context, the recruitment of TLRs contributes to inflammation by amplifying pro-inflammatory cytokine and other mediator release. TLRs thus play a central role in inflammation's impact on neuronal cell health and their participation in neuroinflammation and neurological diseases is an area of growing interest, encompassing not only acute and chronic neurodegenerative disorders15,16,32,33,34,48, but also neuropathic pain49 and even mood disorders50. ATP appears to be one of the most powerful stimuli to enhance and accelerate IL-1β release, via activation of P2X7R and caspase 122,23. Plasma membrane damage or cell death can liberate large amounts of ATP, e.g. following spinal cord injury51. Further, IL-1β release from the spinal cord dorsal horn upon application of ATP only occurred if preceded by an LPS priming stimulus and was P2X7R-dependent52. ATP- and P2X7R-dependent IL-1β release from endotoxin-primed macrophages and microglial cell lines has been known for some time22, but rarely described in CNS primary microglia (cf. ref. 26). We now show, for the first, that this process occurs in rat primary microglia obtained from cortex, spinal cord and cerebellum, using not only LPS, but also the TLR2 ligand zymosan and Pam3CSK4 and the TLR3 ligand poly(I:C). Zymosan activates macrophages via TLR2, in cooperation with TLR6 and CD1435. Pam3CSK4, a synthetic triacylated lipopeptide (LP) that mimics the acylated amino terminus of bacterial LPs, is a potent activator of the pro-inflammatory transcription factor nuclear factor-κB53. Activation is mediated by the interaction between TLR2 and TLR1 which recognizes LPs with three fatty acids, a structural characteristic of bacterial LPs35. Poly(I:C) is a synthetic analogue of double-stranded RNA, a molecular pattern associated with viral infection and is recognized by TLR336. While the TLRs were originally described according to their ability to respond to exogenous microbial products there is sufficient literature to indicate that endogenous products activate TLRs during sterile inflammation. TLR4 recognizes not only LPS, but also ligands called damage associated molecular patterns, released by the injured tissue13,14,54, while oxidized phospholipid-induced inflammation in macrophages is mediated by TLR255. The activation of multiple TLR isoforms in nervous system pathology, coupled with elevated ATP levels in the extracellular milieu and subsequent P2X7R activation may represent an important route for microglia-derived IL-1β. Enriched astrocytes and microglia (greater than 93% GFAP-positive and 95% F4/80-positive, respectively) reportedly express also TLR7 and TLR9 (flow cytometry) and respond to TLR7 and TLR9 agonists56, although a microglial contribution to the astrocyte responses was not excluded. Given that TLR7 and/or TLR9 involvement in neuropathic pain and neurodegenerative disease remains open, we focused on TLR2, -3 and -4 in this study.
Nominally microglia-free astrocytes appeared to be devoid of detectable IL-1β induction/release, suggesting either a helper function of microglia to enable astrocytes to produce IL-1β or to microglia themselves as the ultimate source of the cytokine. Although ‘clean’ astrocytes were not capable of mounting an IL-1β release (and induction at all) they are a target of IL-1β actions, in development and in responses to injury14,56,57. However, using a two-chamber culture system, LPS-stimulated microglia influenced neither IL-1β release nor its intracellular accumulation in purified astrocytes. In addition, culture medium from microglia-depleted, LPS-treated cortical astrocytes was not sufficient to provoke activation of limiting numbers of microglia26. Further, enriched astrocytes incubated with TLR ligands showed no detectable IL-1β immunoreactivity, in contrast to microglia treated with TLR ligand (see also ref. 21). Our current (Fig. 5) and previous data26 clearly point to an interaction between these two cell populations, as microglia respond more robustly in the presence of astrocytes (Fig. 5; see also ref. 26]. If astrocytes do not react to TLR stimulation at all, lack of IL-1β production would not be surprising. However, LPS, zymosan and poly(I:C) up-regulate TLR2 mRNA expression in Leu-Leu-OMe-treated astrocytes (Marinelli and Skaper, unpublished observations; see also ref. 58 and 59) – suggesting as well that Leu-Leu-OMe does not compromise astrocyte functionality – as observed by others42.
The role of stress-responsive kinases in ATP-dependent release of IL-1β from TLR agonist-primed microglia has not been explored. In the present study, release of this cytokine appeared to be sensitive to inhibitors of the stress-activated protein kinases p38 MAPK and JNK. Examination of the intracellular levels of IL-1β, however, revealed that unlike the P2X7R antagonist A740003, neither SB202190 nor SP600125 blocked the ATP-dependent reduction in IL-1β content, but rather the intracellular pool of IL-1β induced by TLR agonist per se - indicating that the effects of these kinase inhibitors were not at the level of P2X7R.
Astrocytes, the predominant CNS cell type also become reactive following injury and have been implicated in the pathogenesis of CNS inflammation56 and neuropathic pain60. To the best of our knowledge, release of IL-1β exclusively from this glial cell type, in a TLR and P2X7R-dependent fashion, has not been reported. Although astrocytes from cortex, spinal cord and cerebellum released IL-1β following priming with TLR2, -3, or -4 activators and ATP incubation, this response was lost upon depletion of the residual microglia population with Leu-Leu-OMe. Intriguingly, cultures of Leu-Leu-OMe-treated cerebellar astrocytes together with added cerebellar microglia displayed a heightened priming response, whereby LPS sensitized the cells to ATP-induced release of IL-1β. Equivalent numbers of microglia alone, however, were essentially devoid of a priming response and IL-1β release. Similar behaviors have been reported also for cortical and spinal cord glia26 and collectively propose the existence of a ‘cross-talk’ pathway between microglia and astrocytes. Such a mechanism in vivo may be an important element in the evolution of an inflammatory pathology, in particular when TLR activation is involved. Together with a growing literature implicating P2X7R up-regulation/activation in nervous system pathology24, this route to IL-1β release may provide opportunities for therapeutic intervention in neuroinflammation-associated disorders.
Methods
Materials
Tissue culture media, antibiotics, fetal calf serum (FCS) and NP40 cell lysis buffer (10×) were obtained from Invitrogen (San Giuliano Milanese, Italy); ATP, LPS (Ultra-Pure LPS-EB from E. coli 0111:B4 strain), zymosan, Pam3CSK4 (VacciGrade™) and polyinosinic-polycytidylic acid (poly(I:C)) (high molecular weight) were from InvivoGen (Cayla-Invivogen Europe, Toulouse, France); SP600125 was from Calbiochem (Merck Millipore, Milan, Italy); poly-L-lysine hydrobromide (mol wt 70,000–150,000), rat tail type I collagen solution, papain, DNase I (bovine pancreas), trypsin inhibitor, Leu-Leu-OMe, SB202190, protease inhibitor cocktail, Pefabloc® SC (100 mM) and all other biochemicals were purchased from Sigma-Aldrich (Milan, Italy) unless noted otherwise; Falcon tissue culture plasticware and culture well inserts (PET membrane, (0.4 μm pore size, were purchased from BD Biosciences (SACCO srl, Cadorago (CO), Italy). Sterilin petri plastic dishes (10 cm Ø) were from Sarstedt (Verona, Italy).
Primary culture of microglia and astrocytes
Mixed glial cell cultures from cortex and spinal cord were prepared from postnatal day 1–2 rat pups (strain: CD) as previously described61. Cerebellar tissue dissociates were prepared from 8-day-old pups, using the protocol described for granule neurons62. Experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and those of the Italian Ministry of Health (D.L. 116/92) and were approved by the Institutional Animal Care and Use Committee. All tissue dissociates were plated in 75-cm2 poly-L-lysine-coated tissue culture flasks at a density of 1.5 brains, 5 spinal cords or 3 cerebella per flask and grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) with 2 mM glutamine, 100 units/ml penicillin/50 μg/ml streptomycin, 50 μg/ml gentamicin and 10% FCS (‘culture medium’). Medium was changed after 24 h and then twice per week. After 7–10 days microglia were dislodged using an orbital shaker (200 rpm for 1 h, 37°C). Culture supernatants (containing mainly microglia) were transferred to plastic Petri dishes (Sterilin) and incubated for 45 min at 37°C (5% CO2, 95% air) to effect differential adhesion of microglia. Adherent microglia (>99.9% purity, flow cytometric analysis) were mechanically scraped into culture medium and replated in this medium on poly-L-lysine-coated microwell culture plates or dishes. The remaining cell monolayers were highly enriched in astrocytes (<5% microglia, flow cytometry using cell type-specific antibodies). In some cases astrocytes were depleted of residual microglia using a 60-min exposure (50 mM) to the lysosomotropic agent Leu-Leu-OMe42, as described previously26.
In some cases cell culture inserts were used to establish astrocyte/microglia co-cultures. Enriched astrocytes were seeded into a poly-L-lysine-coated 24-well plate (3 × 105 cells per well) in culture medium. Twenty-four hours later some cultures were treated with 50 mM Leu-Leu-OMe for 60 min, as described above. In parallel, 24-well culture inserts were seeded with 5 × 104 microglia in culture medium (0.4 ml per insert) and placed in a 24-well plate (notched for inserts) in this same medium (0.8 ml/well)63. Transwell cell culture inserts are convenient, easy-to-use permeable support devices; the suspended design allows for undamaged co-culturing of cells in the lower compartment. The porous transwell membrane allows for communication between the chambers and for passage of microglia-derived factors to the lower chamber containing astrocytes and vice versa. The following day, inserts were transferred to the 24-well plate of astrocytes. The distance between the astrocyte monolayer and microglia on the insert membrane is 1 mm, according to the manufacturer's description. At this time LPS (100 ng/ml final) was added to either the upper or lower chamber (0.4 ml and 0.8 ml final volume, respectively) and incubation continued for another 24 h. The culture medium was then collected and cells lysed, as described below. The IL-1β content of culture supernatants and lysates was determined by ELISA, as described below.
RT-PCR
Cerebellar astrocytes were seeded in poly-lysine-coated 24-well plates at a density of 250,000 cells per well, using culture medium and allowed to adhere overnight. Cells were then incubated with 50 mM Leu-Leu-OMe for 1 h and then replaced with fresh culture medium. Twenty-four hours later total RNA was extracted from cells by TRIzol (Invitrogen), according to the manufacturer's instructions. RNA integrity and quantity were determined by RNA 6000 Nano assay in an Agilent BioAnalyser. RT was performed with Superscript III reverse transcriptase (Invitrogen). The Real-time RT-PCR reaction was performed as described previously26. The following primer pairs were used:
GAPDH, 5′-CAAGGTCATCCATGACAACTTTG-3′ F and 5′-GGGCCATCCACAGTCTTCTG-3′ R (89 bp);
Iba1, 5′-AACTGGAGGCCTTCAAGACG-3′ F and 5′-AACCCCAAGTTTCTCCAGCA-3′ R (101 bp).
Amounts of each gene product were calculated using linear regression analysis from standard curves, demonstrating amplification efficiencies ranging from 90 to 100%. Dissociation curves were generated for each primer pair, showing single product amplification.
Immunofluorescence
Enriched cortical astrocytes were cultured on poly-L-lysine-coated 8-well chamber slides (Labtek) and incubated the following day with 10 ng/ml LPS for 24 h. The cells were then fixed with 4% paraformaldehyde for 30 min at 4°C and washed 4 × 5 min with PBS/0.05% Triton X-100 and blocked with PBS/10% FCS for 1 h at room temperature. The cells were then processed for immunostaining with primary antibodies against the microglial marker F4/80 antigen64 (Abcam ab6640, rat monoclonal, 1:500), the astrocyte marker GFAP (Abcam ab7260, rabbit polyclonal, 1:800), or IL-1β (rabbit polyclonal, 1:250, sc7884, Santa Cruz Biotechnology). The IL-1β antibody used detects both the pro- and mature forms of the cytokine. Cells were then washed 5 × 5 min with PBS and incubated for 1 h at room temperature with anti-mouse-AlexaFluor555 (red) or anti-rabbit-AlexaFluor488 (green) secondary antibody (1:500, Invitrogen). Chamber slides were mounted beneath glass slides using Fluoromount-G (Southern Biotech, USA) and images were acquired on a Leica DMI4000 B microscope equipped for immunofluorescence (Leica Microsystems GmbH, Wetzlar, Germany) using a Leica DFC 480 digital camera.
IL-1β production and release
Microglia and astrocytes were plated in 96-well plates (poly-L-lysine coated; collagen for spinal cord astrocytes) at a density of 100,000 or 50,000 cells per well, respectively, using culture medium and allowed to adhere overnight. These plating densities do not affect glial cell vitality/function66,65. Cells were primed by pre-treating with 1 μg/ml LPS21,28 or other TLR ligands as indicated (optimal concentration of TLR activator chosen from preliminary experiments) for 2 h in serum-free culture medium prior to stimulation with 5 mM ATP28 for 1 h. Neither the TLR ligands used nor ATP affected cell viability. It should be pointed out that commercial sources of LPS are frequently contaminated by other bacterial components, such as lipoproteins, capable of activating both TLR2 and TLR4. The Ultra-Pure LPS-EB preparation used here only activates TLR4 (InvivoGen). None of the TLR ligands or ATP, at the concentrations tested, affected cell viability (data not shown; see also ref. 58). Cell supernatants were collected and stored at −20°C until the day of assay (avoiding repeated freeze-thaw cycles). Cell lysates were prepared by adding to each 96-well culture 100 μl lysis solution containing: 89 μl NP40 lysis buffer, 10 μl of 10× protease inhibitor cocktail and 1 μl of 100 mM Pefabloc SC. IL-1β content of culture medium and cell lysates was analyzed using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Antigenix America, Huntington Station, NY, USA). The IL-1β ELISA assay kit does not distinguish between the inactive 33-kDa precursor (pro-IL-1β) and the bioactive 17-kDa mature form (as is the case for all commercially available kits). Standards with known amounts of IL-1β were used to convert values into absolute concentrations of IL-1β in pg/ml.
Statistics
Data are given as mean ± SEM. Statistical analyses to determine group differences were performed either by two-sample equal variance Student's t test, or by one-way analysis of variance, followed by Dunnett's or Bonferroni's post-hoc tests for comparisons involving more than two data groups. Significance was taken at p < 0.05.
References
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Iadecola, C. & Anrather, J. Stroke research at a crossroad: asking the brain for directions. Nat. Neurosci. 14, 1363–1368 (2011).
Meraz-Ríos, M. A., Toral-Rios, D., Franco-Bocanegra, D., Villeda-Hernández, J. & Campos-Peña, V. Inflammatory processes in Alzheimer's disease. Front. Integr. Neurosci. 7, 59; 10.3389/fnint.2013.00059 (2013).
Najjar, S., Pearlman, D. M., Alper, K., Najjar, A. & Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflammation 10, 43; 10.1186/1742-2094-10-43 (2013).
Tenorio, G., Kulkarni, A. & Kerr, B. J. Resident glial cell activation in response to perispinal inflammation leads to acute changes in nociceptive sensitivity: implications for the generation of neuropathic pain. Pain 154, 71–81 (2013).
Rivat, C. et al. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain 150, 358–368 (2010).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Jensen, T. S. et al. A new definition of neuropathic pain. Pain 152, 2204–2205 (2011).
Colton, C. A. & Wilcock, D. M. Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets 9, 174–191 (2010).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immuno. 11, 373–384 (2010).
Town, T., Jeng, D., Alexopoulou, L., Tan, J. & Flavell, R. A. Microglia recognize double-stranded RNA via TLR3. J. Immunol. 176, 3804–3812 (2006).
Drouin-Ouellet, J. & Cicchetti, F. Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol. Sci. 33, 542–551 (2012).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Bianchi, M. E. & Manfredi, A. A. Immunology. Dangers in and out. Science 323, 1683–1684 (2009).
Stridh, L. et al. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. J. Neurosci. 33, 12041–12051 (2013).
Yao, L. et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J. Neuroinflammation 10, 23; 10.1186/1742-2094-10-23 (2013).
Basu, A., Krady, J. K. & Levison, S. W. Interleukin-1: a master regulator of neuroinflammation. J. Neurosci. Res. 78, 151–156 (2004).
Allan, S. M., Tyrrell, P. J. & Rothwell, N. J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 5, 629–640 (2005).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012).
Tanaka, S. et al. Activation of microglia induces symptoms of Parkinson's disease in wild-type, but not in IL-1 knockout mice. J. Neuroinflammation 10, 143; 10.1186/1742-2094-10-143 (2013).
Chauvet, N. et al. Rat microglial cells secrete predominantly the precursor of interleukin-1β in response to lipopolysaccharide. Eur. J. Neurosci. 14, 609–617 (2001).
Ferrari, D. et al. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176, 3877–3883 (2006).
Walsh, J. G., Muruve, D. A. & Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 15, 84–97 (2014).
Volonté, C., Apolloni, S., Skaper, S. D. & Burnstock, G. P2X7 receptors: channels, pores and more. CNS Neurol. Disord. Drug Targets 11, 705–721 (2012).
Lai, A. Y., Dhami, K. S., Dibal, C. D. & Todd, K. G. Neonatal rat microglia derived from different brain regions have distinct activation responses. Neuron Glia Biol. 7, 5–16 (2011).
Barbierato, M. et al. Astrocyte-microglia cooperation in the expression of a pro-inflammatory phenotype. CNS Neurol. Disord. Drug Targets 12, 608–618 (2013).
Van Dam, A. M., Bauer, J., Tilders, F. J. & Berkenbosch, F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience 65, 815–826 (1995).
Sanz, M. & Di Virgilio, F. Kinetics and mechanism of ATP-dependent IL-1β release from microglial cells. J. Immunol. 164, 4893–4898 (2000).
Kim, D. et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 282, 14975–14983 (2007).
Obata, K. et al. Toll like receptor 3 contributes to spinal glial activation and tactile allodynia after nerve injury. J. Neurochem. 105, 2249–2259 (2008).
Okun, E. et al. Toll-like receptors in neurodegeneration. Brain Res. Rev. 59, 278–292 (2009).
Casula, M. et al. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179, 233–243 (2011).
Wang, Y. et al. Protection of ischemic post conditioning against transient focal ischemia-induced brain damage is associated with inhibition of neuroinflammation via modulation of TLR2 and TLR4 pathways. J. Neuroinflammation 11, 15; 10.1186/1742-2094-11-15 (2014).
Li, X. Q. et al. Role of the TLR4 pathway in blood-spinal cord barrier dysfunction during the bimodal stage after ischemia/reperfusion injury in rats. J Neuroinflammation 11, 62; 10.1186/1742-2094-11-62. (2014).
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97, 13766–13771 (2000).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Honore, P. et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 319, 1376–1385 (2006).
Saura, J. Microglial cells in astroglial cultures: a cautionary note. J. Neuroinflammation 4, 26; 10.1186/1742-2094-4-26 (2007).
Ciccarelli, R. et al. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia 29, 202–211 (2000).
Solà, C., Casal, C., Tusell, J. M. & Serratosa, J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur. J. Neurosci. 16, 1275–1283 (2002).
Thiele, D. L., Kurosaka, M. & Lipsky, P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, l-leucine methyl ester. J. Immunol. 131, 2282–2290 (1983).
Hamby, M. E., Uliasz, T. F., Hewett, S. J. & Hewett, J. A. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J. Neurosci. Methods 150, 128–137 (2006).
Guillemin, G. et al. Obtention and characterization of primary astrocyte and microglial cultures from adult monkey brains. J. Neurosci. Res. 49, 576–591 (1997).
Hewett, S. J. Interferon-gamma reduces cyclooxygenase-2-mediated prostaglandin E2 production from primary mouse astrocytes independent of nitric oxide formation. J. Neuroimmunol. 94, 134–143 (1999).
Streit, W. J., Mrak, R. E. & Griffin, W. S. Microglia and neuroinflammation: a pathological perspective. J. Neuroinflammation 1, 14; 10.1186/1742-2094-1-14 (2004).
Rothwell, N. J. The role of cytokines in neurodegeneration. In: Cytokines in the Nervous System, Edited by Rothwell, N. J. New York: Springer; 1996:145–162.
Perry, V. H. & Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 61, 217–224 (2014).
Jin, J. J., Kim, H. D., Maxwell, J. A., Li, L. & Fukuchi, K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation 5, 23; 10.1186/1742-2094-5-23 (2008).
Nicotra, L., Loram, L. C., Watkins, L. R. & Hutchinson, M. R. Toll-like receptors in chronic pain. Exp. Neurol. 234, 316–329 (2012).
Hajebrahimi, B. et al. The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int. J. Psychiatry Clin. Pract. 18, 41–44 (2014).
Peng, W. et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 106, 12489–12493 (2009).
Clark, A. K. et al. P2X7-dependent release of interleukin-1β and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 30, 573–582 (2010).
Aliprantis, A. O. et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 (1999).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Kadl, A. et al. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 51, 1903–1909 (2011).
Butchi, N. B., Du, M. & Peterson, K. E. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by asreocytes and microglia. Glia 58, 650–664 (2010).
John, G. R., Lee, S. C., Song, X., Rivieccio, M. & Brosnan, C. F. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia 49, 161–176 (2005).
Holm, T. H., Draeby, D. & Owens, T. Microglia are required for astroglial toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia 60, 630–638 (2012).
Carpentier, P. A. et al. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49, 360–374 (2005).
Chen, M. J. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60, 1660–1670 (2012).
Skaper, S. D., Argentini, C. & Barbierato, M. Culture of neonatal rodent microglia, astrocytes and oligodendrocytes from cortex and spinal cord. Methods Mol. Biol. 846, 67–77 (2012).
Facci, L. & Skaper, S. D. Culture of rat cerebellar granule neurons and application to identify neuroprotective agents. Methods Mol. Biol. 846, 23–37 (2012).
Facci, L. & Skaper, S. D. Central nervous system neuron-glia co-culture models. Methods Mol. Biol. 846, 79–89 (2012).
Zhang, D. et al. Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J. Neuroinflammation 8, 3; 10.1186/1742-2094-8-3 (2011).
Culbert, A. A. et al. MAPKAP kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity: Relevance to neuroinflammation in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 281, 23658–23667 (2006).
Skaper, S. D. et al. P2X7 receptors on microglial cells mediate injury to cortical neurons in vitro. Glia 54, 234–242 (2006).
Acknowledgements
Laura Facci was the a recipient of a fellowship from the Fondazione CARIPARO "Progetto Dottorati di Ricerca" Anno 2009. This study was supported in part by MIUR, PON ‘Ricerca e Competitività 2007–2013’ project PON01_02512.
Author information
Authors and Affiliations
Contributions
M.B. carried out the RT-PCR analyses and participated in immunocytochemistry experiments and preparation of spinal cord glia cultures. L.F. carried the culture treatments and nitric oxide and cytokine analyses and participated in preparation of cortical and cerebellar glia cultures and in the statistical analysis. C.A. and C.M. participated in preparation of spinal cord glia cultures and immunocytochemistry experiments. P.G. participated in drafting the manuscript. S.D.S. conceived and coordinated the study and drafted the manuscript. All authors critically revised and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Facci, L., Barbierato, M., Marinelli, C. et al. Toll-Like Receptors 2, -3 and -4 Prime Microglia but not Astrocytes Across Central Nervous System Regions for ATP-Dependent Interleukin-1β Release. Sci Rep 4, 6824 (2014). https://doi.org/10.1038/srep06824
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06824
This article is cited by
-
Crosstalk Between Autophagy and Inflammation in Chronic Cerebral Ischaemia
Cellular and Molecular Neurobiology (2023)
-
Anti-amnesic and Neuroprotective Potential of Genistein Against Alzheimer’s Disease
Revista Brasileira de Farmacognosia (2023)
-
Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: a groundbreaking cell-free approach
Stem Cell Research & Therapy (2022)
-
Astrocyte Mitochondria in White-Matter Injury
Neurochemical Research (2021)
-
Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling
Journal of Neuroinflammation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.