Key Points
-
The complement system provides essential protection against infections but also contributes to the severity of autoimmune diseases
-
The complement system is activated in many rheumatic diseases, as evident from deposition of activation fragments in affected tissue, decreased residual complement function and increased levels of circulating activated complement fragments
-
Although complement is activated in rheumatic diseases, this finding does not mean that complement has a major role in the clinical presentation
-
Systemic complement inhibition is feasible and reasonably safe when the right precautions are taken
-
Complement inhibition has not yet become a common therapeutic strategy for rheumatic disease
Abstract
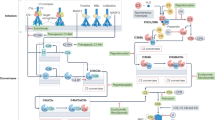
Complement activation is associated with common rheumatic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and systemic vasculitis. Evidence linking complement activation to these diseases includes the presence of complement deposition in affected tissues, decreased levels of complement proteins and high levels of complement activation fragments in the blood and/or synovial fluid of patients with these diseases, as well as data from experimental models. Eculizumab, a monoclonal antibody that inhibits the complement component C5, is now approved for the treatment of rare conditions involving complement hyperactivation, and the success of this therapy has renewed interest in understanding the utility of complement inhibition in rheumatological practice, particularly for SLE. For example, inhibiting C5 is a potential means of reducing glomerular inflammation in lupus nephritis or treating thrombotic microangiopathy in SLE. The complement system is one of multiple mediators of tissue injury in complex diseases such as SLE, and identifying the disease context in which complement activation has a predominant role is a challenge. An added difficulty in RA is identifying a role for therapeutic complement inhibition within the diverse treatment modalities already available. In this Review, evidence for the therapeutic potential of complement manipulation in rheumatology practice is evaluated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Grumach, A. S. & Kirschfink, M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol. Immunol. 61, 110–117 (2014).
Ram, S., Lewis, L. A. & Rice, P. A. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin. Microbiol. Rev. 23, 740–780 (2010).
Baines, A. C. & Brodsky, R. A. Complementopathies. Blood Rev. 31, 213–223 (2017).
Nester, C. M. et al. Atypical aHUS: state of the art. Mol. Immunol. 67, 31–42 (2015).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Hillmen, P. et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 85, 553–559 (2010).
Hill, A., DeZern, A. E., Kinoshita, T. & Brodsky, R. A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 3, 17028 (2017).
Hillmen, P. et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 355, 1233–1243 (2006).
Volokhina, E. J., et al. Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome.. Clin. Immunol. 160, 237–243 (2015).
Sturfelt, G. & Truedsson, L. Complement in the immunopathogenesis of rheumatic disease. Nat. Rev. Rheumatol. 8, 458–468 (2012).
McInnes, I. B. & Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 (2017).
Walport, M. J., Davies, K. A., Morley, B. J. & Botto, M. Complement deficiency and autoimmunity. Ann. NY Acad. Sci. 815, 267–281 (1997).
Kotimaa, J. et al. Sex matters: systemic complement activity of female C57BL/6J and BALB/cJ mice is limited by serum terminal pathway components. Mol. Immunol. 76, 13–21 (2016).
Okroj, M., Heinegard, D., Holmdahl, R. & Blom, A. M. Rheumatoid arthritis and the complement system. Ann. Med. 39, 517–530 (2007).
Holers, V. M. Targeting mechanisms at sites of complement activation for imaging and therapy. Immunobiology 221, 726–732 (2016).
Brodeur, J. P., Ruddy, S., Schwartz, L. B. & Moxley, G. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 34, 1531–1537 (1991).
Corvetta, A. et al. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin. Exp. Rheumatol. 10, 433–438 (1992).
Hogasen, K. et al. Terminal complement pathway activation and low lysis inhibitors in rheumatoid arthritis synovial fluid. J. Rheumatol. 22, 24–28 (1995).
Jose, P. J., Moss, I. K., Maini, R. N. & Williams, T. J. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann. Rheum. Dis. 49, 747–752 (1990).
Morgan, B. P., Daniels, R. H. & Williams, B. D. Measurement of terminal complement complexes in rheumatoid arthritis. Clin. Exp. Immunol. 73, 473–478 (1988).
Moxley, G. & Ruddy, S. Elevated C3 anaphylatoxin levels in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 28, 1089–1095 (1985).
Swaak, A. J. et al. An analysis of the levels of complement components in the synovial fluid in rheumatic diseases. Clin. Rheumatol. 6, 350–357 (1987).
Kaplan, R. A. et al. Metabolism of C4 and factor B in rheumatoid arthritis. Relation to rheumatoid factor. Arthritis Rheum. 23, 911–920 (1980).
Kemp, P. A. et al. Immunohistochemical determination of complement activation in joint tissues of patients with rheumatoid arthritis and osteoarthritis using neoantigen-specific monoclonal antibodies. J. Clin. Lab. Immunol. 37, 147–162 (1992).
Trouw, L. A., Rispens, T. & Toes, R. E. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 331–339 (2017).
Suwannalai, P. et al. Low-avidity anticitrullinated protein antibodies (ACPA) are associated with a higher rate of joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 73, 270–276 (2014).
Trouw, L. A. et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 60, 1923–1931 (2009).
Happonen, K. E. et al. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 62, 3574–3583 (2010).
Melin Furst, C. et al. The C-type lectin of the aggrecan G3 domain activates complement. PLoS ONE 8, e61407 (2013).
Melin Furst, C. et al. Quantitative mass spectrometry to study inflammatory cartilage degradation and resulting interactions with the complement system. J. Immunol. 197, 3415–3424 (2016).
Sjoberg, A., Onnerfjord, P., Morgelin, M., Heinegard, D. & Blom, A. M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308 (2005).
Sjoberg, A. P. et al. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol. Immunol. 46, 830–839 (2009).
Trouw, L. A., Blom, A. M. & Gasque, P. Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 45, 1199–1207 (2008).
Van Schravendijk, M. R. & Dwek, R. A. Interaction of C1q with DNA. Mol. Immunol. 19, 1179–1187 (1982).
Jiang, H. X., Siegel, J. N. & Gewurz, H. Binding and complement activation by C-reactive protein via the collagen-like region of C1q and inhibition of these reactions by monoclonal antibodies to C-reactive protein and C1q. J. Immunol. 146, 2324–2330 (1991).
Banda, N. K. et al. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J. Immunol. 183, 5928–5937 (2009).
Mehta, G., Scheinman, R. I., Holers, V. M. & Banda, N. K. A new approach for the treatment of arthritis in mice with a novel conjugate of an anti-C5aR1 antibody and C5 siRNA. J. Immunol. 194, 5446–5454 (2015).
Wang, Y., Rollins, S. A., Madri, J. A. & Matis, L. A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl Acad. Sci. USA 92, 8955–8959 (1995).
Banda, N. K. et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J. Immunol. 177, 1904–1912 (2006).
Banda, N. K., Takahashi, K., Wood, A. K., Holers, V. M. & Arend, W. P. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J. Immunol. 179, 4101–4109 (2007).
Ji, H. et al. Arthritis critically dependent on innate immune system players. Immunity 16, 157–168 (2002).
Banda, N. K. et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 188, 1469–1478 (2012).
Banda, N. K. et al. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor MAp44 expression. J. Immunol. 193, 2455–2468 (2014).
Katschke, K. J. Jr et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J. Exp. Med. 204, 1319–1325 (2007).
Macor, P. et al. Treatment of experimental arthritis by targeting synovial endothelium with a neutralizing recombinant antibody to C5. Arthritis Rheum. 64, 2559–2567 (2012).
Durigutto, P. et al. Prevention of arthritis by locally synthesized recombinant antibody neutralizing complement component C5. PLoS ONE 8, e58696 (2013).
Blom, A. M., Nandakumar, K. S. & Holmdahl, R. C4b-binding protein (C4BP) inhibits development of experimental arthritis in mice. Ann. Rheum. Dis. 68, 136–142 (2009).
Macedo, A. C. & Isaac, L. Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway. Front. Immunol. 7, 55 (2016).
Taylor, P. R. et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192, 359–366 (2000).
Manderson, A. P., Botto, M. & Walport, M. J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22, 431–456 (2004).
Martin, M. & Blom, A. M. Complement in removal of the dead — balancing inflammation. Immunol. Rev. 274, 218–232 (2016).
Nauta, A. J. et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 32, 1726–1736 (2002).
Crow, M. K. & Kirou, K. A. Interferon-alpha in systemic lupus erythematosus. Curr. Opin. Rheumatol. 16, 541–547 (2004).
Lood, C. et al. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 60, 3081–3090 (2009).
Son, M., Santiago-Schwarz, F., Al-Abed, Y. & Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl Acad. Sci. USA 109, E3160–E3167 (2012).
Gomes, R. C. et al. Features of 847 childhood-onset systemic lupus erythematosus patients in three age groups at diagnosis: a Brazilian multicenter study. Arthritis Care Res. (Hoboken) 68, 1736–1741 (2016).
Lintner, K. E. et al. Early components of the complement classical activation pathway in human systemic autoimmune diseases. Front. Immunol. 7, 36 (2016).
Leffler, J., Bengtsson, A. A. & Blom, A. M. The complement system in systemic lupus erythematosus: an update. Ann. Rheum. Dis. 73, 1601–1606 (2014).
Dragon-Durey, M. A., Blanc, C., Marinozzi, M. C., van Schaarenburg, R. A. & Trouw, L. A. Autoantibodies against complement components and functional consequences. Mol. Immunol. 56, 213–221 (2013).
Trendelenburg, M., Marfurt, J., Gerber, I., Tyndall, A. & Schifferli, J. A. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 42, 187–188 (1999).
Trouw, L. A. et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J. Clin. Invest. 114, 679–688 (2004).
Bao, L., Cunningham, P. N. & Quigg, R. J. Complement in lupus nephritis: new perspectives. Kidney Dis. (Basel) 1, 91–99 (2015).
Sekine, H. et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J. Immunol. 166, 6444–6451 (2001).
Jennette, J. C. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Cornec, D., Cornec-Le Gall, E., Fervenza, F. C. & Specks, U. ANCA-associated vasculitis — clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 12, 570–579 (2016).
Falk, R. J., Terrell, R. S., Charles, L. A. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl Acad. Sci. USA 87, 4115–4119 (1990).
Chen, M., Kallenberg, C. G. & Zhao, M. H. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat. Rev. Nephrol. 5, 313–318 (2009).
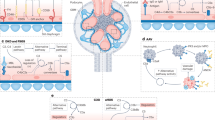
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
Chen, M., Jayne, D. R. W. & Zhao, M. H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat. Rev. Nephrol. 13, 359–367 (2017).
Xing, G. Q. et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J. Clin. Immunol. 29, 282–291 (2009).
Sethi, S. et al. Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol. Dial. Transplant. 32, i139–i145 (2017).
Yuan, J. et al. C5a and its receptors in human anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Arthritis Res. Ther. 14, R140 (2012).
Gou, S. J., Yuan, J., Chen, M., Yu, F. & Zhao, M. H. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 83, 129–137 (2013).
Camous, L. et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 117, 1340–1349 (2011).
Leffler, J. et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188, 3522–3531 (2012).
Wang, H., Wang, C., Zhao, M. H. & Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 181, 518–527 (2015).
Bekker, P. et al. Characterization of pharmacologic and pharmacokinetic properties of CCX168, a potent and selective orally administered complement 5a receptor inhibitor, based on preclinical evaluation and randomized phase 1 clinical study. PLoS ONE 11, e0164646 (2016).
Robinson, W. H. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 580–592 (2016).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Struglics, A. et al. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res. Ther. 18, 223 (2016).
Morgan, B. P., Boyd, C. & Bubeck, D. Molecular cell biology of complement membrane attack. Semin. Cell Dev. Biol. http://dx.doi.org/10.1016/j.semcdb.2017.06.009 (2017).
Bradley, K. et al. Synthesis of classical pathway complement components by chondrocytes. Immunology 88, 648–656 (1996).
Ritter, S. Y. et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 65, 981–992 (2013).
Lepus, C. M. et al. Brief report: carboxypeptidase B serves as a protective mediator in osteoarthritis. Arthritis Rheumatol. 66, 101–106 (2014).
Bloom, A. C. et al. Deletion of the membrane complement inhibitor CD59a drives age and gender-dependent alterations to bone phenotype in mice. Bone 84, 253–261 (2016).
Chimenti, M. S., Ballanti, E., Triggianese, P. & Perricone, R. Vasculitides and the complement system: a comprehensive review. Clin. Rev. Allergy Immunol. 49, 333–346 (2015).
Jachiet, M. et al. The clinical spectrum and therapeutic management of hypocomplementemic urticarial vasculitis: data from a French nationwide study of fifty-seven patients. Arthritis Rheumatol. 67, 527–534 (2015).
Mahler, M., van Schaarenburg, R. A. & Trouw, L. A. Anti-C1q autoantibodies, novel tests, and clinical consequences. Front. Immunol. 4, 117 (2013).
Wisnieski, J. J. & Jones, S. M. IgG autoantibody to the collagen-like region of Clq in hypocomplementemic urticarial vasculitis syndrome, systemic lupus erythematosus, and 6 other musculoskeletal or rheumatic diseases. J. Rheumatol. 19, 884–888 (1992).
Strait, R. T. et al. IgG1 protects against renal disease in a mouse model of cryoglobulinaemia. Nature 517, 501–504 (2015).
Trendelenburg, M. et al. The role of complement in cryoglobulin-induced immune complex glomerulonephritis. J. Immunol. 175, 6909–6914 (2005).
Hirt-Minkowski, P. et al. A trial of complement inhibition in a patient with cryoglobulin-induced glomerulonephritis. Case. Rep. Nephrol. Urol. 2, 38–45 (2012).
Cicardi, M., Suffritti, C., Perego, F. & Caccia, S. Novelties in the diagnosis and treatment of angioedema. J. Investig. Allergol. Clin. Immunol. 26, 212–221 (2016).
Montgomery, R. A. et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am. J. Transplant. 16, 3468–3478 (2016).
Pham, H., Santucci, S., Yang, W. H. Successful use of daily intravenous infusion of C1 esterase inhibitor concentrate in the treatment of a hereditary angioedema patient with ascites, hypovolemic shock, sepsis, renal and respiratory failure. Allergy Asthma Clin. Immunol. http://dx.doi.org/10.1186/s13223-014-0062-9 (2014).
Emmens, R. W. et al. On the value of therapeutic interventions targeting the complement system in acute myocardial infarction. Transl Res. 182, 103–122 (2017).
Caliezi, C. et al. C1-esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol. Rev. 52, 91–112 (2000).
Wong, E. K. & Kavanagh, D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl Res. 165, 306–320 (2015).
Benamu, E. & Montoya, J. G. Infections associated with the use of eculizumab: recommendations for prevention and prophylaxis. Curr. Opin. Infect. Dis. 29, 319–329 (2016).
Swaak, A. J. et al. Determination of the half-life of C3 in patients and its relation to the presence of C3-breakdown products and/or circulating immune complexes. Rheumatol. Int. 2, 161–166 (1982).
Pascual, M. et al. Metabolism of complement factor D in renal failure. Kidney Int. 34, 529–536 (1988).
Ricklin, D. & Lambris, J. D. New milestones ahead in complement-targeted therapy. Semin. Immunol. 28, 208–222 (2016).
Holers, V. M. et al. New therapeutic and diagnostic opportunities for injured tissue-specific targeting of complement inhibitors and imaging modalities. Semin. Immunol. 28, 260–267 (2016).
Fridkis-Hareli, M. et al. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood 118, 4705–4713 (2011).
Atkinson, C. et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation 131, 1171–1180 (2015).
Grant, E. P. et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J. Exp. Med. 196, 1461–1471 (2002).
Vergunst, C. E. et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 46, 1773–1778 (2007).
Steinsson, K., Erlendsson, K. & Valdimarsson, H. Successful plasma infusion treatment of a patient with C2 deficiency and systemic lupus erythematosus: clinical experience over forty-five months. Arthritis Rheum. 32, 906–913 (1989).
Mehta, P. et al. SLE with C1q deficiency treated with fresh frozen plasma: a 10-year experience. Rheumatology (Oxford) 49, 823–824 (2010).
Arkwright, P. D., Riley, P., Hughes, S. M., Alachkar, H. & Wynn, R. F. Successful cure of C1q deficiency in human subjects treated with hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 133, 265–267 (2014).
Olsson, R. F. et al. Allogeneic hematopoietic stem cell transplantation in the treatment of human C1q deficiency: the Karolinska experience. Transplantation 100, 1356–1362 (2016).
Castellano, G. et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 103, 3813–3820 (2004).
Schwaeble, W. et al. Follicular dendritic cells, interdigitating cells, and cells of the monocyte-macrophage lineage are the C1q-producing sources in the spleen. Identification of specific cell types by in situ hybridization and immunohistochemical analysis. J. Immunol. 155, 4971–4978 (1995).
van Schaarenburg, R. A. et al. The production and secretion of complement component C1q by human mast cells. Mol. Immunol. 78, 164–170 (2016).
Bermea, R. S., Sharma, N., Cohen, K. & Liarski, V. M. Use of eculizumab in atypical hemolytic uremic syndrome, complicating systemic lupus erythematosus. J. Clin. Rheumatol. 22, 320–323 (2016).
El-Husseini, A. et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am. J. Kidney Dis. 65, 127–130 (2015).
Raufi, A. G. et al. Atypical hemolytic uremic syndrome secondary to lupus nephritis, responsive to eculizumab. Hematol. Rep. 8, 6625 (2016).
Pickering, M. C. et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology (Oxford) 54, 2286–2288 (2015).
Coppo, R. et al. Dramatic effects of eculizumab in a child with diffuse proliferative lupus nephritis resistant to conventional therapy. Pediatr. Nephrol. 30, 167–172 (2015).
Strakhan, M. et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case. Rep. Hematol. 2014, 704371 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01029587 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02994927 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01363388 (2016).
Jayne, D. R. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2016111179 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02384317 (2017).
Rosenblad, T. et al. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr. Nephrol. 29, 2225–2228 (2014).
Prohaszka, Z., Nilsson, B., Frazer-Abel, A. & Kirschfink, M. Complement analysis 2016: clinical indications, laboratory diagnostics and quality control. Immunobiology 221, 1247–1258 (2016).
Beurskens, F. J., van Schaarenburg, R. A. & Trouw, L. A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 68, 6–13 (2015).
Seelen, M. A. et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J. Immunol. Methods 296, 187–198 (2005).
Nilsson, B. & Ekdahl, K. N. Complement diagnostics: concepts, indications, and practical guidelines. Clin. Dev. Immunol. 2012, 962702 (2012).
Blom, A. M., Osterborg, A., Mollnes, T. E. & Okroj, M. Antibodies reactive to cleaved sites in complement proteins enable highly specific measurement of soluble markers of complement activation. Mol. Immunol. 66, 164–170 (2015).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
Sciascia, S. et al. Expanding the therapeutic options for renal involvement in lupus: eculizumab, available evidence. Rheumatol. Int. http://dx.doi.org/10.1007/s00296-017-3686-5 (2017).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.C.P. declares that he is a scientific adviser for Alexion Pharmaceuticals, Achillion and Ra Pharmaceuticals. A.M.B. declares that she is listed as an inventor on the patent application “Antibodies specific for complement component C4d and uses thereof”. L.A.T. declares no competing interests.
Rights and permissions
About this article
Cite this article
Trouw, L., Pickering, M. & Blom, A. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol 13, 538–547 (2017). https://doi.org/10.1038/nrrheum.2017.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.125
This article is cited by
-
Seven-chain adaptive immune receptor repertoire analysis in rheumatoid arthritis reveals novel features associated with disease and clinically relevant phenotypes
Genome Biology (2024)
-
Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer
BioDrugs (2023)
-
Brief report on the relation between complement C3a and anti dsDNA antibody in systemic lupus erythematosus
Scientific Reports (2022)
-
Lupus nephritis with corticosteroid responsiveness: molecular changes of CD46-mediated type 1 regulatory T cells
Pediatric Research (2022)
-
Association of C1q gene cluster variants with rheumatoid arthritis: a pilot study
Rheumatology International (2022)