Abstract
Purpose
To evaluate the relationships between baseline visual field (VF) mean deviation (MD) and subsequent progression in treated glaucoma.
Methods
Records of patients seen in a glaucoma practice between 1999 and 2009 were reviewed. Patients with glaucomatous optic neuropathy, baseline VF damage, and ≥8 SITA-standard 24-2 VF were included. Patients were divided into tertiles based upon baseline MD. Automated pointwise linear regression determined global and localized rates (decibels (dB) per year) of change. Progression was defined when two or more adjacent test locations in the same hemifield showed a sensitivity decline at a rate of >1.0 dB per year, P<0.01.
Results
For mild, moderate, and severe groups, progression was noted in 29.5, 31.2, and 26.0% of eyes (P=0.50) and global rates of VF change of progressing eyes were −1.3±1.2, −1.01±0.7, and −0.9±0.5 dB/year (P=0.09, analysis of variance). Within these groups, intraocular pressure (IOP) in stable vs progressing eyes were 15.5±3.3 vs 17.0±3.1 (P<0.01), 15.4±3.3 vs 15.9±2.5 (P=0.28), and 14.0±2.8 vs 14.8±2.3 mm Hg (P=0.07). More glaucoma filtering surgeries were performed in eyes with worse MD. There was no significant difference between groups regarding their risk of progression in both univariate (P=0.50) and multivariate (P=0.26) analyses adjusting for differences in follow-up IOP.
Conclusions
After correcting for differences in IOP in treated glaucoma patients, we did not find a relationship between the rate of VF change (dB per year) and the severity of the baseline VF MD. This finding may have been due to more aggressive IOP lowering in eyes with more severe disease. Eyes with lower IOP progressed less frequently across the spectrum of VF loss.
Similar content being viewed by others
Introduction
Glaucoma is a multifactorial neurodegenerative disorder characterized by progressive structural and functional injury of the optic nerve complex (optic nerve and parapapillary region) for which intraocular pressure (IOP) remains the only proven modifiable risk factor. The major randomized clinical trials in glaucoma have provided important information regarding disease progression across the glaucoma spectrum, as well as a better understanding of other risk factors for visual field (VF) progression.1, 2, 3, 4, 5, 6, 7 For instance, a 20% reduction of baseline IOP in the Ocular Hypertension Treatment Study reduced the rate of progression from 9.5% in the observation group to 4.4% in treated ocular hypertensives.1 In the Early Manifest Glaucoma Trial (EMGT), which studied a population with newly diagnosed glaucoma, progression occurred in 59% of treated vs 76% of control patients.8 Based on these and other studies, older age, decreased central corneal thickness (CCT), lower ocular perfusion pressure, disc hemorrhage, and baseline status of the VF and optic nerve were identified as variables associated with glaucoma VF progression.1, 2, 3, 4, 5, 6, 7, 8
It remains unclear as to whether eyes with more advanced baseline VF damage have a faster rate of VF sensitivity decline measured in decibels (dB) per year. The Advanced Glaucoma Intervention Study (AGIS) found that non-progressing eyes had better AGIS VF scores at 4 years than progressing eyes,9 whereas the OHTS reported that each 0.2 dB greater VF pattern standard deviation (PSD) at baseline increased the risk of progression by 27%.10 In the EMGT, eyes presenting with a baseline mean deviation (MD) worse than −4.0 dB progressed faster (P=0.05) than eyes with better MD.8
Randomized, prospective, clinical trials are the ideal method to test a hypothesis. However, relevant clinical information can also be obtained from retrospective studies that include large population samples seen in clinical practice.11, 12, 13, 14, 15, 16, 17, 18, 19 In the present study, we sought to investigate the relationship between baseline VF MD, IOP, and trend analysis VF progression outcomes in eyes with treated, established glaucoma.
Materials and methods
We included subjects from the New York Glaucoma Progression Study (GAPS) evaluated in the glaucoma referral practice of the authors (JML, RR, and CT) from January 1999 to September 2009. At baseline assessment, all patients underwent complete ophthalmologic examination, standard achromatic perimetry (24-2 SITA-SAP, Humphrey Field Analyzer II, Carl Zeiss Meditec, Inc., Dublin, CA, USA), and optic disc stereo photographs, patients were reexamined, usually at 3–6 months intervals, and the same tests repeated were within 6–12 months. The study was approved by the New York Eye and Ear Infirmary Institutional Review Board and followed the tenets of the Declaration of Helsinki.
Since a greater number of VF tests increases the sensitivity of pointwise linear regression (PLR) analysis in detecting progression,20, 21 we selected all GAPS subjects with glaucomatous optic neuropathy, repeatable VF loss and ≥8 SITA-standard VF examinations. We chose this minimum VF number based on recent studies that investigated the sensitivity and specificity of trend analysis and which demonstrated that this would be a satisfactory number of tests to detect significant VF change.21
GAPS methodology has been previously reported.22 In short, all eyes had a best-corrected baseline visual acuity of 20/40 and a spherical equivalent refractive error less than six diopters. If both eyes of the same patient were eligible, the eye with the greater number of VF tests was enrolled.
VF analysis
A glaucomatous VF was defined as a glaucoma hemifield test outside normal limits or if the PSD was triggered at P<0.05 on at least two consecutive baseline VF tests. The two baseline tests required reliability indices better than 25% in order to be included. We divided the patients into three equally sized groups after ranking their baseline VF MD values: mild, MD better than −4.20 dB; moderate, MD between −4.21 and −8.17 dB; severe, worse than −8.17 dB.
Automated PLR analysis was performed using Progressor software (Version 3.3, Medisoft, Inc., Leeds, UK) providing slopes (dB per year) of progression both globally and locally for each point based on threshold maps, as well as its level of significance (P-values). Details of the software have been described elsewhere.23 All patients were familiar with achromatic automated perimetry prior to enrollment. Progression was defined as the presence of a test point with a slope of sensitivity over time >1.0 dB loss per year, with P<0.01. For edge points (nasal-most points of the 24-2), a stricter slope criterion of >2.0 dB loss per year (also with P<0.01) was used. Because using a single progressing point meeting the aforementioned criteria could result in high false-positive rates,24 we increased the specificity of our analysis by requiring that at least two progressing points had to be adjacent and within the same hemifield in order to denote the eye as progressing.22 The MD values of the baseline VF were recorded.
Clinical data
We applied standard definitions of IOP peak, mean, and fluctuation.22 Peak IOP was defined as the highest IOP measurement during follow-up. The mean IOP was the average of all pressure measurements obtained after the date of the first VF test analysed in the regression. IOP fluctuation was defined as the SD of the mean values. We excluded all IOP measurements within 4 weeks of any type of incisional or laser surgery to minimize the role of transitory IOP changes that may occur in this period. CCT was measured using ultrasonic pachymetry (DGH-550, DGH Technology Inc., Exton, PA, USA).
Statistical analysis
Analysis of variance (ANOVA) with Bonferroni post hoc correction was used for comparisons of continuous variables among subgroups. Analysis of covariance (ANCOVA) was used for comparisons after adjusting for differences among subgroups. Pearson's correlation coefficients (r) were calculated to assess the relationship between MD, IOP parameters, and rates of VF sensitivity change measured in dB per year. Logistic regression adjusted to follow-up time was performed to test the association between baseline VF MD and reaching a progression end point. Statistical significance was defined at P<0.05 in the final model. Computerized statistical analysis was performed using MedCalc (MedCalc, Inc., Mariakerke, Belgium).
Results
The mean MD values of the baseline VF tests for the mild (n=196), moderate (n=195), and severe (n=196) were −2.4±1.2, −6.1±1.2, and −12.9±4.3 dB, respectively (P<0.01). There were no significant differences among groups regarding mean age, follow-up time, number of VF tests, and glaucoma subtypes (P>0.05). Table 1 compares the demographic and clinical characteristics of the three subgroups. There were more individuals of African and Hispanic origin in the severe group (P=0.03). Notably, eyes with severe baseline VF damage had thinner central corneas, lower mean and peak IOP, and a slower rate of VF change (dB/year). After adjusting for these differences, there was no significant difference in global rates of VF change (dB/year) among the groups (P>0.20, ANCOVA).
Even though there was a weak but significant trend, suggesting that eyes with worse MD progressed more slowly (Pearson's r=−0.08, P=0.03), this association became non-significant after adjusting for the mean follow-up IOP (P=0.37). More glaucoma filtering surgeries were performed in eyes with worse MD (mild, 46; moderate, 73; severe, 87; P<0.01).
Univariable logistic regression revealed no association between baseline VF MD and reaching a progression end point (OR=1.01, 95% CI=0.96–1.3, P=0.86). After adjusting for statistically significant differences found in the univariable model for mean IOP, CCT, age, follow-up time, and having filtering surgery, there was still no association between baseline VF MD and progression (OR=0.99, 95% CI=0.95–1.03, P=0.86). Because aggressive IOP reduction following filtering surgery was more common in the severe group, we performed a subanalysis only in eyes that never underwent a filtering procedure (n=381). In a logistic regression adjusting for mean follow-up IOP, there was no significant association between the baseline MD and a progression end point (OR=0.97, P=0.26). Similarly, there was no correlation between global rates of change (dB per year) and baseline MD adjusted for mean IOP (Pearson's r=−0.14, P=0.63).
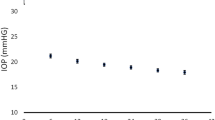
We further divided each of the groups into three equally sized subgroups based on the mean follow-up IOP (IOP tertiles). For all glaucoma stages, maintaining the mean IOP in the lower range was associated with decreased risk of progression (Figure 1). Similarly, eyes with higher mean follow-up IOP showed faster rates of global VF change (dB per year) than those with lower values (Figure 2). Global rates of VF change (dB/year) of progressing eyes for the mild, moderate, and severe groups were −1.3±1.2, −1.01±0.7, and −0.9±0.5 dB per year, respectively (P=0.09, ANOVA). Mean follow-up IOPs in the stable vs progressing eyes were 15.5±3.3 vs 17.0±3.1 (P<0.01), 15.4±3.3 vs 15.9±2.5 (P=0.28), and 14.0±2.8 vs 14.8±2.3 mm Hg, respectively (P=0.07) (Figure 3). Also, in all stages, eyes that presented with peak IOP ≥18 mm Hg progressed almost twice as fast as those which did not present any IOP peaks above this value (Figure 4).
Rate (%) of eyes that reached a progression end point based on the baseline visual field (VF) damage and mean treated intraocular pressure (IOP).  , 1st IOP tertile;
, 1st IOP tertile;  , 2nd IOP tertile;
, 2nd IOP tertile;  , 3rd IOP tertile. Mean IOP values for each tertile: mild: <14.68, 14.68–17.45, and 17.45–23.81 mm Hg; moderate: <14.50, 14.50–16.80, and 16.80–22.81 mm Hg; severe: <13.31, 13.31–15.35, and 15.35–21.58 mm Hg.
, 3rd IOP tertile. Mean IOP values for each tertile: mild: <14.68, 14.68–17.45, and 17.45–23.81 mm Hg; moderate: <14.50, 14.50–16.80, and 16.80–22.81 mm Hg; severe: <13.31, 13.31–15.35, and 15.35–21.58 mm Hg.
Rates of global visual field (VF) change based on the baseline level of VF damage and mean follow-up intraocular pressure (IOP).  , 1st IOP tertile;
, 1st IOP tertile;  , 2nd IOP tertile;
, 2nd IOP tertile;  , 3rd IOP tertile. Mean IOP values for each tertile: mild: <14.68, 14.68–17.45, and 17.45–23.81 mm Hg; moderate: <14.50, 14.50–16.80, and 16.80–22.81 mm Hg; severe: <13.31, 13.31–15.35, and 15.35–21.58 mm Hg.
, 3rd IOP tertile. Mean IOP values for each tertile: mild: <14.68, 14.68–17.45, and 17.45–23.81 mm Hg; moderate: <14.50, 14.50–16.80, and 16.80–22.81 mm Hg; severe: <13.31, 13.31–15.35, and 15.35–21.58 mm Hg.
Discussion
We investigated the association between baseline VF MD, rates of VF change (dB per year), and incidence of VF progression (% of cases) in treated glaucoma. We found that the baseline status of the VF did not affect the rates of VF change or the likelihood of reaching a progression end point.
There are at least two potential reasons—not mutually exclusive—why patients first come to the office with severe VF loss. The first is that the disease has been progressing at an average rate (dB per year), but may have been for a long time undetected because of insufficient or infrequent eye examination(s). The second is that there is indeed a distribution of speed of deterioration (dB per year), with some patients progressing more rapidly than others. One inference that comes from our study is that regardless of why patients show up with severe disease, substantial IOP lowering therapy is an effective means of slowing the velocity of progression (dB per year) and the number of eyes reaching progression end points (%). However, perhaps there are some high-risk patients showing rapid rates of VF deterioration who may continue to progress despite aggressive therapy. It is also important to note that eyes with more severe VF loss require more aggressive therapy to slow rates of progression (dB/year) because little visual function remains so that any amount of loss represents a greater percentage of loss of the remaining vision.
This study has several findings that are relevant to clinical practice. Clinicians commonly estimate the risk of progression and treat to prevent blindness based on certain baseline variables, such as age, life expectancy, CCT, IOP, and baseline level of structural and functional damage. Many physicians assume that, all other parameters being equal, eyes with worse baseline VF injury are more likely to progress faster than those with less baseline damage. In clinical practice, however, this does not appear to be the case. The present study showed that in a referral glaucoma practice, where patients are treated at the clinician's discretion and not following a standardized treatment protocol, given similar IOP, the rate of VF change (dB per year) is similar, regardless of the extent of baseline VF MD.
It is worth noting that among all subgroups, progressing eyes had higher IOP levels than stable eyes. One possible explanation as to why the mean IOP difference between progressing and non-progressing eyes was not statistically different in the moderate and severe group could be that additional IOP-independent factors may have a more important role on progression among eyes that, as we demonstrated, had their IOP lowered more aggressively (ie, a greater likelihood of surgery). Figure 1 also demonstrates this trend among severely damaged eyes. In this group, there was not much difference in the risk of progression between eyes in the second and third mean IOP tertiles, while eyes in the lowest IOP tertile had a significantly lower risk of progression. This confirms that among patients with severe baseline VF damage, substantial IOP reduction (mean IOP in lower teens) may be necessary to slow rates of progression (dB per year). Another possible explanation could be a ‘floor’ effect in eyes with severe VF damage, which may have affected the PLR. That is, among eyes with worse threshold sensitivities, deterioration may occur up to a point and thereafter the regression slope may be falsely flattened. We also found that even among eyes that never underwent glaucoma filtering surgery, there was no association between baseline MD and progression, even after adjusting for mean follow-up IOP. The use of event analysis would still be limited by the ‘floor’ effect, as one would be unable to detect change once a point has reached its lowest sensitivity. An alternative approach would be using percentage change of VF sensitivity, as has been described using the Guided Progression Analysis (Carl Zeiss Meditec, Inc.). However, there is currently no commercially available method that provides these measurements in a pointwise, localized manner as proposed by the present study.
Peak IOP appears to be a significant, easily measured parameter that may influence the rate of progression both in dB per year and the incidence of eyes reaching progression end points (%). For instance, based on our results, if a patient presents with severe baseline MD (average=−12.9 dB, Table 1) and the mean treated IOP is kept above 13.35 mm Hg (Figure 1), almost 30% of those patients will progress, and their average rate of progressive VF loss will be approximately −0.9 dB per year. Considering an average patient taken from our sample (aged 67 years), this patient may lose 4.5 and 9.0 dB from his global threshold sensitivity in the next 5 and 10 years, respectively. The Los Angeles Latino Eyes Study suggested that even smaller amounts of VF loss as measured by MD can lead to meaningful functional disability.25 Assuming an MD<−15 dB for significant functional disability, the risk that this hypothetical patient will be visually impaired in the next 5–10 years is very high. Given that today in the United States, individuals aged 67 years have an average life expectancy ranging between 17 and 20 years (http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_09.pdf), our hypothetical patient could certainly live long enough to become blind from glaucoma.
This study has certain limitations. First, this is a retrospective cohort of a tertiary care service and our results may not be generalizable. Second, we did not perform diurnal curve monitoring, which might have provided a better understanding of IOP variability and effectiveness of therapy. Third, using global indices that summarize the VF results may not precisely describe the status of VF loss. The localized vs diffuse nature of VF loss is not differentiated by global indices. For instance, patients with similar baseline MD but with damage extending to both hemifields were shown to progress faster that those with single hemifield damage.26 We did not use the baseline PSD in this study as it increases as the VF worsens, and then decreases in cases of very severe damage, which could have affected our results. Also, in the Los Angeles Latino Eye Study, MD values were shown to correlate well with quality of life and quality of vision.25 Notably, we used the status of achromatic perimetry to define baseline glaucomatous damage, even though we agree that VF status alone underestimates the amount of retinal ganglion cell damage.27
Our results should not be interpreted as guidelines of how to treat or to determine target IOP for glaucoma patients. The fact that almost 30% of eyes reached our predefined progression end point stresses that therapy could have been more intense and that observed IOP values could have been lowered further. Moreover, we did not adjust our analyses for treatment effectiveness and compliance as there are currently no objective methods of measuring these confounding factors. Yet, this is the first descriptive analysis showing the relationship between baseline VF status, IOP, and progression in a real-world population.
Unlike most of the major clinical trials, we used trend analysis to define and measure progression rates (dB per year) and progression end points (%) based on PLR criteria. Nouri-Mahdavi et al9 used a similar method to evaluate VF progression in part of the population followed in the AGIS. The present study used a selected cohort with a large number of VFs, which increases the sensitivity and specificity of PLR in detecting significant VF change.20, 21 This method also allowed us not only to determine the incidence of progression (%) in different groups, but also the velocity of progressive VF loss (dB per year). Even though we understand that the assumption of linearity of VF progression during the entire follow-up may be questionable, rates of VF change (dB per year) allow clinicians to look retrospectively at how patients behaved under the past therapy as well as to estimate their future status assuming that other factors (modifiable or not) remain unchanged.
Our main finding that a worse MD at baseline was not associated with faster progression should not be interpreted to mean that these patients do not require enhanced therapy. As highlighted previously, the observation that their global rates of VF change (dB per year) and overall incidence of progression (%) did not differ from other groups was mainly attributed to more aggressive therapy in severe cases, which is recommended and often done in clinical practice. One key message from this cohort is that lowering IOP and minimizing peak IOP is effective in maintaining rates of VF change at similar levels across the glaucoma spectrum.

References
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–713.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M . Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–1279.
AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130: 490–491.
Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA et al. CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001; 108: 1943–1953.
Miglior S, Torri V, Zeyen T, Pfeiffer N, Vaz JC, Adamsons I . Intercurrent factors associated with the development of open-angle glaucoma in the European glaucoma prevention study. Am J Ophthalmol 2007; 144 (2): 266–275.
Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN . Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol 2003; 136: 805–813.
Drance S, Anderson DR, Schulzer M . Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001; 131: 699–708.
Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z, EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007; 114: 1965–1972.
Nouri-Mahdavi K, Hoffman D, Gaasterland D, Caprioli J . Prediction of visual field progression in glaucoma. Invest Ophthalmol Vis Sci 2004; 45: 4346–4351.
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 714–720.
Cioffi GA, Liebmann JM . Translating the OHTS results into clinical practice. J Glaucoma 2002; 11: 375–377.
Singh K . The randomized clinical trial: beware of limitations. J Glaucoma 2004; 13: 87–89.
Jampel HD . We should treat fewer patients with elevated intraocular pressure now that we know the results of the Ocular Hypertension Treatment Study. Arch Ophthalmol 2004; 122: 378–379.
Wishart PK . Interpretation of the glaucoma ‘landmark studies’. Br J Ophthalmol 2009; 93: 561–562.
Kass MA, Gordon MO, Kymes SM . Incorporating the results of the Ocular Hypertension Treatment Study into clinical practice. Arch Ophthalmol 2005; 123: 1021–1022.
Kent DM, Kitsios G . Against pragmatism: on efficacy, effectiveness and the real world. Trials 2009; 10: 48.
Tunis SR, Stryer DB, Clancy CM . Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290: 1624–1632.
Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA . Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care 2005; 43: 551–557.
De Moraes CG, Ritch R, Liebmann JM . Bridging the major prospective National Eye Institute-sponsored glaucoma clinical trials and clinical practice. J Glaucoma 2011; 20: 1–2.
Chauhan BC, Garway-Heath DF, Goñi FJ, Rossetti L, Bengtsson B, Viswanathan AC et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol 2008; 92 (4): 569–573.
Spry PG, Bates AB, Johnson CA, Chauhan BC . Simulation of longitudinal threshold visual field data. Invest Ophthalmol Vis Sci 2000; 41 (8): 2192–2200.
De Moraes CG, Juthani V, Liebmann JM, Teng CC, Tello C, Susanna R et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 2010 (in press).
Fitzke FW, Hitchings RA, Poinoosawmy D, McNaught AI, Crabb DP . Analysis of visual field progression in glaucoma. Br J Ophthalmol 1996; 80: 40–48.
Gardiner SK, Crabb DP . Examination of different pointwise linear regression methods for determining visual field progression. Invest Ophthalmol Vis Sci 2002; 43: 1400–1407.
McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP, Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am J Ophthalmol 2007; 143: 1013–1023.
De Moraes CG, Prata TS, Tello C, Ritch R, Liebmann JM . Glaucoma with early visual field loss affecting both hemifields and the risk of disease progression. Arch Ophthalmol 2009; 127: 1129–1134.
Quigley HA, Dunkelberger GR, Green WR . Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 1989; 107: 453–464.
Acknowledgements
This study was supported in part by the Irvin Kessler Research Fund of the New York Glaucoma Research Institute, New York, NY, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This material was presented as a poster at ARVO 2010.
Rights and permissions
About this article
Cite this article
Forchheimer, I., de Moraes, C., Teng, C. et al. Baseline mean deviation and rates of visual field change in treated glaucoma patients. Eye 25, 626–632 (2011). https://doi.org/10.1038/eye.2011.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.33
Keywords
This article is cited by
-
Persistent severe visual field impairment is associated with obesity and tumour invasiveness, but not with pituitary dysfunction, in patients with craniopharyngioma
Endocrine (2023)
-
Association of macular structure, function, and vessel density with foveal threshold in advanced glaucoma
Scientific Reports (2022)
-
Testing the eligibility of glaucoma patients for potential gene therapy among a clinic population
International Ophthalmology (2022)
-
Long-term treatment success and safety of combined phacoemulsification plus excimer laser trabeculostomy: an 8-year follow-up study
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Visual field progression in patients with severe glaucoma
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)





 , 1st IOP tertile;
, 1st IOP tertile;  , 2nd IOP tertile.
, 2nd IOP tertile.
 , 1st IOP tertile;
, 1st IOP tertile;  , 2nd IOP tertile.
, 2nd IOP tertile.