Abstract
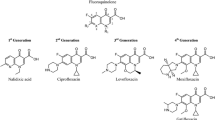
Acute bacterial conjunctivitis, the most common cause of conjunctivitis, is responsible for approximately 1% of all primary-care consultations. Of the topical ophthalmic antibiotics used to treat acute bacterial conjunctivitis, fluoroquinolones are especially useful because they possess a broad antibacterial spectrum, are bactericidal in action, are generally well tolerated, and have been less prone to development of bacterial resistance. Besifloxacin, the latest advanced fluoroquinolone approved for treating bacterial conjunctivitis, is the first fluoroquinolone developed specifically for topical ophthalmic use. It has a C-8 chlorine substituent and is known as a chloro-fluoroquinolone. Besifloxacin possesses relatively balanced dual-targeting activity against bacterial topoisomerase IV and DNA gyrase (topoisomerse II), two essential enzymes involved in bacterial DNA replication, leading to increased potency and decreased likelihood of bacterial resistance developing to besifloxacin. Microbiological data suggest a relatively high potency and rapid bactericidal activity for besifloxacin against common ocular pathogens, including bacteria resistant to other fluoroquinolones, especially resistant staphylococcal species. Randomized, double-masked, controlled clinical studies demonstrated the clinical efficacy of besifloxacin ophthalmic suspension 0.6% administered three-times daily for 5 days to be superior to the vehicle alone and similar to moxifloxacin ophthalmic solution 0.5% for bacterial conjunctivitis. In addition, besifloxacin ophthalmic suspension 0.6% administered two-times daily for 3 days was clinically more effective than the vehicle alone for bacterial conjunctivitis. Besifloxacin has also been shown in preclinical animal studies to be potentially effective for the “off-label” treatment of infections following ocular surgery, prophylaxis of endophthalmitis, and the treatment of bacterial keratitis. Taken together, clinical and preclinical animal studies indicate that besifloxacin is an important new option for the treatment of ocular infections.
Similar content being viewed by others
References
McDonnell PJ. How do general practitioners manage eye disease in the community? Br J Ophthalmol. 1988;72:733–736.
Diamant JI, Hwang DG. Therapy for bacterial conjunctivitis. Ophthalmol Clin North Am. 1999;12:15–20.
Rose P. Management strategies for acute infective conjunctivitis in primary care: a systematic review. Expert Opin Pharmacother. 2007;8:1903–1921.
Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis (Review). Cochrane Database Syst Rev. 2006;2:CD001211.
Høding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5–17.
Morrow GL, Abbott RL. Conjunctivitis. Am Fam Physician. 1998;57:735–746.
Drug Facts and Comparisons 2008. St. Louis, MO: Wolters Kluwer Health; 2008:1764–1765.
Besivance (package insert). Rochester, NY: Bausch & Lomb Inc.; April 2009.
Zymaxid (package insert). Irvine, CA: Allergan, Inc.; May 2010.
Kaliamurthy J, Nelson Jesudasan CA, Geraldine P, Parmar P, Kalawathy CM, Thomas PA. Comparison of in vitro susceptibilities of ocular bacterial isolates to gatifloxacin and other topical antibiotics. Ophthalmic Res. 2005;37:117–122.
Hwang DG. Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fluoroquinolones. Surv Ophthalmol. 2004;49:S79–S83.
Cambau E, Matrat S, Xiao-Su P, et al. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009;63:443–450.
Chen FJ, Lo HJ. Molecular mechanisms of fluoroquinolone resistance. J Microbiol Immunol Infect. 2003;36:1–9.
O’Brien TP. Evidence-based review of moxifloxacin. Int Ophthalmol Clin. 2006;46:61–72.
Schlech BA, Blondeau J. Future of ophthalmic antiinfective therapy and the role of moxifloxacin ophthalmic solution 0.5% (Vigamox®). Surv Ophthalmol. 2005;50:S64–S67.
McDonald MD, Blondeau JM. Emerging antibiotic resistance in ocular infections and the role of fluoroquinolones. J Cataract Refract Surg. 2010; 36:1588–1598
Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–958.
Asbell PA, Sahm DF, Shaw M, Draghi DC, Brown NP. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg. 2008;34:814–818.
Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115:51–56.
Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms — results from the ARMOR 2009 surveillance study. Am J Ophthalmol. 2011;152:567–574.
Chalita MR, Hofling-Lima AL, Paranhos A, Schor P, Belfort R. Shifting trends in in vitro antibiotic susceptibilities for common ocular isolates during a period of 15 years. Am J Ophthalmol. 2004;137:43–51.
Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctivitis isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249:111–119.
Miller D, Flynn PM, Scott IU, Alfonso EC, Flynn HW. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Ophthalmol. 2006;124:479–483.
Ward KH, Lepage J-F, Driot J-Y. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J Ocul Pharmacol Ther. 2007;23:243–256.
Zhang J-Z, Cavet ME, Ward KW. Anti-inflammatory effects of besifloxacin, a novel fluoroquinolone, in primary human corneal epithelial cells. Curr Eye Res. 2008;33:923–932.
Protzko E, Bowman L, Abelson M, Shapiro A; for the AzaSite Clinical Study Group. Phase 3 safety comparisons for 1.0% azithromycin in polymeric mucoadhesive eye drops versus 0.3% tobramycin eye drops for bacterial conjunctivitis. Invest Ophthalmol Vis Sci. 2007;48:3425–3429.
Blondeau JM, Borsos S, Hesje CK. Antimicrobial efficacy of gatifloxacin and moxifloxacin with and without benzalkonium chloride compared with ciprofloxacin and levofloxacin against methicillin-resistant Staphylococcus aureus. J Chemother. 2007;19:146–151.
Mah FS, Romanowski EG, Kowalski RP, Yates Ka, Gordon YJ. Benzalkonium chloride (BAK) significantly enhances the antibacterial efficacy of gatifloxacin in the Staphylococcus aureus NZW rabbit keratitis model. Invest Ophthalmol Vis Sci. 2006;47: E-Abstract 1905, ARVO Meeting. Available at: http://abstracts.iovs.org//cgi/content/abstract/47/5/1905?sid=6efc2603-26f1-4d5f-827b-27ba1c7d3199. Accessed May 17 2012.
Kowalski RP, Kowalski BR, Romanowski EG, Mah FS, Thompson PP, Gordon YJ. The in vitro impact of moxifloxacin and gatifloxacin concentration (0.5% vs 0.3%) and the addition of benzalkonium chloride on antibacterial efficacy. Am J Ophthalmol. 2006;142:730–735.
Mah FS, Romanowski EG, Kowalski RP, Yates KA, Gordon YJ. Does topical 0.3% gatifloxacin need BAK (benzalkonium chloride) to treat gatifloxacinresistant, methicillin-resistant Staphylococcus epidermidis in the NZW rabbit keratitis model? Invest Ophthalmol Vis Sci. 2007;48: E-abstract 4743 (ARVO Abstract). Available at: http://abstracts.iovs.org//cgi/content/abstract/48/5/4743?sid=4f898b23-a3b5-4b0b-a24bf8fd898c8b5f. Accessed May 17 2102.
Haas W, Gearinger LS, Usner DW, et al. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin opthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin Ophthalmol. 2011;5:1369–1379.
Haas W, Pillar CM, Zurenko GE, et al. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53:3552–3560.
Haas W, Pillar C, Hesje CK, Sanfilippo CM, Morris TW. Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. J Antimicrob Chemother. 2010;65:1441–1447.
Haas W, Pillar CM, Hesje CK, Sanfilippo CM, Morris TW. In vitro time-kill experiments with besifloxacin, moxifloxacin and gatifloxacin in the absence and presence of benzalkonium chloride. J Antimicrob Chemother. 2011;66:840–844.
Sanfilippo CM, Hesje C, Haas W, Morris TW. Topoisomerase mutations that are associated with high-level resistance to earlier fluoroquinolones in Staphylococcus aureus have less effect on the antibacterial activity of besifloxacin. Chemotherapy. 2011;57:363–371.
Shinabarger DL, Zurenko GE, Hesje CK, Sanfilippo CM, Morris TW, Haas W. Evaluation of the effect of bacterial efflux pumps on the antibacterial activity of the novel fluoroquinolone besifloxacin. J Chemother. 2011;23:80–86.
Proksch JW, Granvil CP, Siou-Mermet R, et al. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J Ocul Pharmacol Ther. 2009;25:335–344.
Torkildsen G, Proksch JW, Shapiro S, Lynch SK, Comstock TL. Concentrations of besifloxacin, gatifloxacin and moxifloxacin in human conjunctiva after topical ocular administration. Clin Ophthalmol. 2010;4:331–341.
Donnenfeld ED, Comstock TL, Proksch JW. Human aqueous humor concentrations of besifloxacin, moxifloxacin, and gatifloxacin after topical ocular application. J Cataract Refract Surg. 2011;37:1082–1089.
Yoshida J, Kim A, Pratzer KA, Stark WJ. Aqueous penetration of moxifloxacin 0.5% ophthalmic solution and besifloxacin 0.6% ophthalmic suspension in cataract surgery patients. J Cataract Refract Surg. 2010;36:1499–1502.
Segreti J, Jones RN, Bertino JS, Jr. Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. J Ocul Pharmacol Ther. 2012;28:3–11.
Comstock TL, Paterno MR, Usner DW, Pichichero ME. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trials. Pediatr Drugs. 2010;12:105–112.
Tepedino ME, Heller WH, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25:1159–1169.
Karpecki P, DePaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31:514–526.
McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116:1615–1623.
Silverstein BE, Allaire C, Bateman KM, Gearinger LS, Morris TW, Comstock TL. Efficacy and tolerability of besifloxacin 0.6% ophthalmic suspension administered twice daily for three days in the treatment of bacterial conjunctivitis: A multicenter, randomized, double-masked, vehicle-controlled, parallel-group study in adults and children. Clin Ther. 2011;33:13–26.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and dose compliance. Clin Ther. 2001;23:1296–1310.
Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: A review of literature. J Behav Med. 2008;31:213–224.
Richter A, Anton SE, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25:2307–2335.
Comstock TL, Paterno MR, DeCory HH, Usner DW. Safety and tolerability of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis: data from six clinical and phase I safety studies. Clin Drug Invest. 2010;30:675–685.
Norcross EW, Sanders ME, Moore Q III, et al. Comparative efficacy of besifloxacin and other fluoroquinolones in a prophylaxis model of penicillin-resistant Streptococcus pneumoniae rabbit endophthalmitis. J Ocul Pharmacol Ther. 2010;26:237–243.
Sanders ME, Moore QC, Norcross EWM, et al. Comparison of besifloxacin, gatifloxacin, and moxifloxacin against strains of Pseudomonas aeruginosa with different quinolone susceptibility patterns in a rabbit model of keratitis. Cornea. 2011;30:83–90.
Michaud L. Efficacy of besifloxacin in the treatment of corneal ulcer. Clin Refract Optom. 2011;22:90–93.
Sanders ME, Norcross EW, Moore QC 3rd, Shafiee A, Marquart ME. Efficacy of besifloxacin in a rabbit model of methicillin-resistant Staphylococcus aureus keratitis. Cornea. 2009;28:1055–1060.
Sanders ME, Moore QC 3rd, Norcross EW, Shafiee A, Marquart ME. Efficacy of besifloxacin in an early treatment model of methicillin-resistant Staphylococcus aureus keratitis. J Ocul Pharmacol Ther. 2010;26:193–198.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Brien, T.P. Besifloxacin Ophthalmic Suspension, 0.6%: a Novel Topical Fluoroquinolone for Bacterial Conjunctivitis. Adv Therapy 29, 473–490 (2012). https://doi.org/10.1007/s12325-012-0027-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-012-0027-7