Abstract
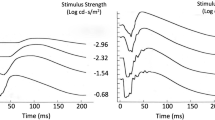
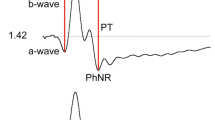
We evaluated the full field electroretinogram (ERG) to assess age-related changes in retina function in humans. ERG recordings were performed on healthy subjects with normal fundus appearance, lack of cataract and 20/20 acuity, aged 20–39 years (n = 27; mean age 25 ± 5, standard deviation), 40–59 years (n = 20; mean 53 ± 5), and 60–82 years (n = 18; mean 69 ± 5). Multiple ERG tests were applied, including light and dark-adapted stimulus-response function, dark adaptation and dynamic of recovery from a single bright flash under dark-adapted conditions. Changes in ERG properties were found in the oldest age group when compared with the two younger age groups. (1) The photopic hill effect was less pronounced. (2) Both photopic a-wave and b-wave amplitudes and implicit times were increased at high stimulus strengths. (3) Dark adaptation time was delayed for pure rod and L/M cone-driven responses, respectively. (4) Dark-adapted a-wave but not b-wave amplitudes were reduced, yielding higher B/A ratios. (5) Dark-adapted a- and b-waves implicit times were prolonged: there was a direct proportional correlation between minimal a-wave implicit times and age. (6) The dynamic of dark current recovery from a bright flash, under dark-adapted conditions, was transiently faster at intervals between 0.9 and 2 s. These results denote that aging of the healthy retina is accompanied by specific functional changes, which must be taken into account to optimally diagnose potential pathologies.






Similar content being viewed by others
References
Gunkel RD, Gouras P (1963) Changes in scotopic visibility thresholds with age. Arch Ophthalmol 69:4–9
Jackson GR, Owsley C, McGwin G (1999) Aging and dark adaptation. Vision Res 39:3975–3982
Jackson GR, Owsley C (2000) Scotopic sensitivity during adulthood. Vision Res 40:2467–2473
McFarland RA, Domey RG, Warren AB, Ward DC (1960) Dark adaptation as a function of age: I. A statistical analysis. J Gerontol 15:267–279
Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC (1993) Symptomatic abnormalities of dark adaptation in patients with age-related Bruch’s membrane change. Br J Ophthalmol 77:549–554
Curcio CA, Millican CL, Allen KA, Kalina RE (1993) Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci 34:3278–3296
Curcio CA, Medeiros NE, Millican CL (1996) Photoreceptor loss in age related macular degeneration. Invest Ophthalmol Vis Sci 37:1236–1249
Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M (1995) Retinal photoreceptor density decreases with age. Ophthalmology 102:1853–1859
Gartner S, Henkind P (1981) Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol 65:23–28
Kilbride PE, Hutman LP, Fishman M, Read JS (1986) Foveal cone pigment density difference in the aging human eye. Vision Res 26:321–325
Newsome DA, Negreiro M (2009) Reproducible measurement of macular light flash recovery time using a novel device can indicate the presence and worsening of macular diseases. Curr Eye Res 34:162–170
Werner JS (1996) Visual problems of the retina during ageing: compensation mechanisms and colour constancy across the life span. Prog Retin Eye Res 15:621–645
Birch DG, Anderson JL (1992) Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol 110:1571–1576
Karpe G, Rickenbach K, Thomasson S (1950) The clinical electroretinogram. I. The normal electroretinogram above fifty years of age. Acta Ophthalmol 28:301–305
Kergoat H, Kergoat MJ, Justino L (2001) Age-related changes in the flash electroretinogram and oscillatory potentials in individuals age 75 and older. J Am Geriatr Soc 49:1212–1217
Martin DA, Heckenlively JR (1982) The normal electroretinogram. Doc Ophthalmol Proc Ser 31:135–144
Peterson H (1968) The normal b-potential in the single flash clinical electroretinogram: a computer technique study of the influence of sex and age. Acta Ophthalmol 99:7–77
Wright CE, Williams DE, Drasdo N, Harding GFA (1985) The influence of age on the electroretinogram and visual evoked potential. Doc Ophthalmol 59:365–384
Zeidler I (1959) The clinical electroretinogram. IX. The normal electroretinogram. Value of the b-potential in different age groups and its differences in men and women. Acta Ophthalmol 37:294–301
Cideciyan AV, Jacobson SG (1996) An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vision Res 36:2609–2621
Chiti Z, North RV, Mortlock KE, Drasdo N (2003) The S-cone electroretinogram: a comparison of techniques, normative data and age-related variation. Ophthalmic Physiol Opt 23:370–376
Nilsson J, Wright T, Westall CA (2008) Rod a-wave analysis using high intensity flashes adds information on rod system function in 25% of clinical ERG recordings. Vision Res 48:1920–1925
Weleber RG (1981) The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci 20:392–399
Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M (2010) Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg 36:308–312
Pokorny J, Smith VC, Lutze M (1987) Aging of the human lens. Appl Opt 26:1437–1440
Sample PA, Esterson FD, Weinreb RN, Boynton RM (1988) The aging lens: in vivo assessment of light absorption in 84 human eyes. Invest Ophthalmol Vis Sci 29:1306–1311
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M, International Society for Clinical Electrophysiology of Vision (2009) ISCEV standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118:69–77
McCulloch DL, Hamilton R (2010) Essentials of photometry for clinical electrophysiology of vision. Doc Ophthalmol 121:77–84
Hamilton R, Bees MA, Chaplin CA, McCulloch DL (2007) The luminance-response function of the human photopic electroretinogram: a mathematical model. Vision Res 47:2968–2972
Ueno S, Kondo M, Niwa Y, Terasaki H, Miyake Y (2004) Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040
Wali N, Leguire LE (1992) The photopic hill: a new phenomenon of the light adapted electroretinogram. Doc Ophthalmol 80:335–342
Pepperberg DR, Birch DG, Hofmann KP, Hood DC (1996) Recovery kinetics of human rod phototransduction inferred from the two-branched alpha-wave saturation function. J Opt Soc Am 13:586–600
Brown PK, Wald G (1964) Visual pigments in single rods and cones of the human retina. Direct measurements reveal mechanisms of human night and color vision. Science 144:45–52
Ethen CM, Feng X, Olsen TW, Ferrington DA (2005) Declines in arrestin and rhodopsin in the macula with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci 46:769–775
Rufiange M, Rousseau S, Dembinska O, Lachapelle P (2002) Cone-dominated ERG luminance-response function: the photopic hill revisited. Doc Ophthalmol 104:231–248
Dornstauder B, Freund P, Gilmour G, Sauvé Y (2010) Wavelet analysis shows age-related changes in full field ERG oscillatory potentials in humans. Invest Ophthal Vis Sci E Abstract 1496
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Suzuki S, Horiguchi M, Tanikawa A, Miyake Y, Kondo M (1998) Effect of age on short-wavelength sensitive cone electroretinogram and long- and middle-wavelength sensitive cone electroretinogram. Jpn J Ophthalmol 42:424–430
Eliasieh K, Liets LC, Chalupa LM (2007) Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci 48:2824–2830
Breton ME, Schueller AW, Lamb TD, Pugh EN Jr (1994) Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 35:295–309
Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT (2002) Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: normal aging, progression with disease, and test-retest variability. Arch Ophthalmol 120:1045–1051
Jackson GR, McGwin G Jr, Phillips JM, Klein R, Owsley C (2004) Impact of aging and age-related maculopathy on activation of the a-wave of the rod-mediated electroretinogram. Invest Ophthalmol Vis Sci 45:3271–3278
Gao H, Hollyfield JG (1992) Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 33:1–17
Keunen JEE, van Norren D, van Meel GJ (1979) Density of foveal cone pigments at older age. Invest Ophthalmol Vis Sci 28:985–991
Elsner AE, Berk L, Burns SA, Rosenberg PR (1988) Aging and human cone photopigments. J Opt Soc Am 5:2106–2112
Acknowledgments
Grants: Alberta Heritage Foundation for Medical Research (AHFMR), the Canadian National Institute for the Blind, the Olive Young Foundation, the Lena McLaughlin Foundation (Mona & Rod McLennan), and the Alberta Centre on Ageing (ACA). PF was a recipient of an AHFMR summer studentship for 2009. JW was a recipient of an ACA studentship for 2008. FG was funded by an ISCEV visiting scientist grant (2008). YS is an AHFMR Senior Scholar. The authors would like to thank Ms Sharee Kuny for her invaluable assistance in editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freund, P.R., Watson, J., Gilmour, G.S. et al. Differential changes in retina function with normal aging in humans. Doc Ophthalmol 122, 177–190 (2011). https://doi.org/10.1007/s10633-011-9273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-011-9273-2