Abstract
Background
Altered regulation of vascular tone and particularly vasospasms are thought to be a risk factor for the progression of primary open angle glaucoma (POAG). Apoptosis of retinal ganglion cells and possibly vascular tone regulation in glaucoma might be caused by oxidative stress. The aim of the present study was to investigate the influence of oxygen free radicals on the tone of ciliary arteries.
Methods
The experiments were carried out with fresh ring preparations from porcine ciliary arteries obtained from a slaughterhouse. The preparations were placed in a self-designed myograph system and were kept under physiologic conditions (pH 7.4, 37°C, Krebs-Henseleit-Buffer, 1.75 mM Ca2+). The muscles were sub-maximally activated by depolarization to −41 mV Nernst potential for K+. The pre-activated preparations were exposed to hydroxyl radicals generated by the Fenton reaction (4 mM H2O2; 30 μM Fe3+). Exposure time varied between 10 s and 60 s in order to obtain different radical-time-doses. The developed force was evaluated relatively to the developed force at maximal depolarization to −4 mV.
Results
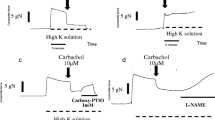
Ten seconds of radical exposure result in an additional increase of the relative developed force from 0.35 ± .08 to 0.62 ± 0.12 (P = 0.003; n = 8). Comparable results were obtained for 20 s and 60 s radical exposures. The developed force of a maximal activation to −4 mV was not reduced after a 10 s radical exposure (0.84 ± 0.13; P = 0.25; n = 5), but was significantly reduced after 20 s exposure (0.25 ± 0.21; P = 0.005; n = 6) and was virtually 0 after 60 s exposure.
Discussion
The data shows that oxygen free radicals induce transient contractions of isolated ciliary artery rings. The shape of these contractions shows parallels to vasospasms. Thus the established system may serve as an in vitro model of vasospasms.




Similar content being viewed by others
References
Aladag MA, Turkoz Y, Ozcan C, Sahna E, Parlakpinar H, Akpolat N, Cigremis Y (2006) Caffeic acid phenethyl ester (CAPE) attenuates cerebral vasospasm after experimental subarachnoidal haemorrhage by increasing brain nitric oxide levels. Int J Dev Neurosci 24:9–14
Auch-Schwelk W, Katusic ZS, Vanhoutte PM (1989) Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension 13:859–864
Broadway DC, Drance SM (1998) Glaucoma and vasospasm. Br J Ophthalmol 82:862–870
Clark JF, Sharp FR (2006) Bilirubin oxidation products (BOXes) and their role in cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26:1223–1233
Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF (2004) Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol 137:62–69
Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E (2002) The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21:359–393
Flammer J, Pache M, Resink T (2001) Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res 20:319–349
Galambos P, Vafiadis J, Vilchez SE, Wagenfeld L, Matthiessen ET, Richard G, Klemm M, Zeitz O (2006) Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 113:1832–1836
Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL (2005) Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 46:877–883
Gherghel D, Hosking SL, Cunliffe IA (2004) Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci 45:3546–3554
Gugleta K, Orgul S, Hasler PW, Picornell T, Gherghel D, Flammer J (2003) Choroidal vascular reaction to hand-grip stress in subjects with vasospasm and its relevance in glaucoma. Invest Ophthalmol Vis Sci 44:1573–1580
Janssen PM, Zeitz O, Hasenfuss G (1999) Transient and sustained impacts of hydroxyl radicals on sarcoplasmic reticulum function: protective effects of nebivolol. Eur J Pharmacol 366:223–232
Ko ML, Peng PH, Ma MC, Ritch R, Chen CF (2005) Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med 39:365–373
Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE (2004) Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med 37:803–812
Mulieri LA, Hasenfuss G, Ittleman F, Blanchard EM, Alpert NR (1989) Protection of human left ventricular myocardium from cutting injury with 2,3-butanedione monoxime. Circ Res 65:1441–1449
Neri S, Signorelli S, Pulvirenti D, Mauceri B, Cilio D, Bordonaro F, Abate G, Interlandi D, Misseri M, Ignaccolo L, Savastano M, Azzolina R, Grillo C, Messina A, Serra A, Tsami A (2006) Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic Res 40:615–618
Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL (2000) Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res 852:326–334
Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA (1990) Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol 68:1581–1589
Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A (2005) Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol 123:458–463
Tezel G (2006) Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 25:490–513
Tutaj M, Brown CM, Brys M, Marthol H, Hecht MJ, Dutsch M, Michelson G, Hilz MJ (2004) Dynamic cerebral autoregulation is impaired in glaucoma. J Neurol Sci 220:49–54
Veriac S, Tissie G, Bonne C (1993) Oxygen free radicals adversely affect the regulation of vascular tone by nitric oxide in the rabbit retina under high intraocular pressure. Exp Eye Res 56:85–88
Wiklund L, McGregor CG, Miller VM (1996) Effects of prolonged exposure to oxygen-derived free radicals in canine pulmonary arteries. Am J Physiol 270:H2184–H2190
Zeitz O, Galambos P, Wagenfeld L, Wiermann A, Wlodarsch P, Praga R, Matthiessen ET, Richard G, Klemm M (2006) Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol 90:1245–1248
Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kogler H, Moller K, Hasenfuss G, Janssen PM (2002) Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na(+)-Ca(2+) exchange. Circ Res 90:988–995
Zeitz O, Schlichting L, Richard G, Strauss O (2006) Lack of antioxidative properties of vitamin C and pyruvate in cultured retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol
Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT (1989) Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem 264:18890–18895
Acknowledgements
This work was supported by a grant of the Ernst und Berta Grimmke Stiftung to Oliver Zeitz and Lars Wagenfeld.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver Zeitz and Lars Wagenfeld, these authors contributed equally.
Rights and permissions
About this article
Cite this article
Zeitz, O., Wagenfeld, L., Wirtz, N. et al. Influence of oxygen free radicals on the tone of ciliary arteries: a model of vasospasms of ocular vasculature. Graefes Arch Clin Exp Ophthalmol 245, 1327–1333 (2007). https://doi.org/10.1007/s00417-006-0526-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0526-9