Abstract
Aims/hypothesis
Fractal analysis of the retinal vasculature provides a global measure of the complexity and density of retinal vessels summarised as a single variable: the fractal dimension. We investigated fractal dimensions as long-term predictors of microvasculopathy in type 1 diabetes.
Methods
We included 180 patients with type 1 diabetes in a 16 year follow-up study. In baseline retinal photographs (from 1995), all vessels in a zone 0.5–2.0 disc diameters from the disc margin were traced using Singapore Institute Vessel Assessment-Fractal image analysis software. Artefacts were removed by a certified grader, and fractal dimensions were calculated using the box-counting method. At follow-up (in 2011), diabetic neuropathy, nephropathy and proliferative retinopathy were assessed and related to baseline fractal dimensions in multiple regressions adjusted for sex and baseline age, diabetes duration, HbA1c, BP, BMI, vibration perception threshold, albuminuria, retinopathy and vessel diameters.
Results
Mean baseline age and diabetes duration were 21.0 and 13.4 years, respectively, and of patients 50.0% were males. The mean fractal dimension was 1.3817. The 16 year incidences of neuropathy, nephropathy and proliferative retinopathy were 10.8%, 8.0% and 27.9%, respectively. Multiple regression analyses showed a lower fractal dimension to significantly predict incident neuropathy (OR 1.17 per 0.01 fractal dimension decrease [95% CI 1.01, 1.36]), nephropathy (OR 1.40 per 0.01 fractal dimension decrease [95% CI 1.10, 1.79]) and proliferative retinopathy (OR 1.22 per 0.01 fractal dimension decrease [95% CI 1.09, 1.37]).
Conclusions/interpretation
The retinal vascular fractal dimension is a shared biomarker of diabetic microvasculopathy, thus indicating a possible common pathogenic pathway. Retinal fractal analysis therefore is a potential tool for risk stratification in type 1 diabetes.
Similar content being viewed by others
Introduction
Microvascular complications remain common in type 1 diabetes [1, 2]. Identifying early biomarkers of diabetic peripheral neuropathy (DPN), diabetic nephropathy (DN) and diabetic retinopathy (DR) will allow the earlier implementation of treatment and support the development of new treatment modalities.
Measurements of vessel calibres in retinal photos could potentially deliver such biomarkers [3–7], but more global variables are available, for example retinal vascular fractal dimensions. The fractal dimension summarises the complexity and density of branching structures characterised by self-similarity and a similar level of complexity despite changes in magnification (for example snowflakes, tree branches, lightning). Correspondingly, the retinal vascular tree has features of a fractal [8]. The fractal dimension increases with increasing structural complexity.
Few studies on retinal vascular fractal dimensions and microvascular complications have been performed. Higher fractal dimensions have been linked to early signs of DR [9] and proliferative diabetic retinopathy (PDR) [10]. However, one study reported lower fractal dimensions in patients with PDR after pan-retinal photocoagulation and in patients with DPN, while a trend towards associations with DN was seen [11]. The latter observation is supported by a study examining the likelihood of microalbuminuria in DN [12]. All studies were of cross-sectional design.
Only a single prospective study exists. This study examined retinal vascular fractal dimensions and incident DR [13]. However, the follow-up period was short (mean 2.9 years) and incident cases had mild non-proliferative DR. Thus, the predictive value of fractal dimensions on severe DR remains unknown.
We carried out a prospective study to examine whether retinal vascular fractal dimensions can predict long-term microvascular complications in type 1 diabetes. We aimed to investigate the predictive value of retinal vascular fractal dimensions on the 16 year incidence of DPN, DN and PDR in a young population-based Danish cohort.
Methods
Study population
This study formed part of a paediatric cohort study of Danish children with type 1 diabetes, initiated in 1987–1989 (n = 720): the Danish Cohort of Pediatric Diabetes 1987. Clinical characteristics of this cohort have been reported elsewhere [14–17]. Of this cohort, 339 participants were included in a baseline examination in 1995, where they also had fundus photographs taken. These 339 participants were thus eligible for 16 year follow-up in 2011 and for fractal analysis. Of these, 15 (4.4%) were excluded from follow-up because of missing baseline retinal photographs, 13 (3.8%) had died and 63 (18.6%) were unreachable because they had emigrated or could not be contacted owing to research protection.
Of the remaining 248 patients, 185 (74.6%) participated in follow-up in 2011 and 63 (25.4%) declined to take part. A dropout analysis of the reduction in the cohort from 1995 to 2011 was described previously [14]. In brief, the 185 participants at follow-up had significantly lower levels of HbA1c, higher values of vibration perception threshold (VPT) and were older than the ones who dropped out. The groups did not differ in terms of sex, age at onset, diabetes duration, BP, BMI, presence of albuminuria, or level of DR at baseline.
The study was performed in accordance with the criteria of the Helsinki II Declaration and was approved by the local scientific ethics committee. All patients gave written informed consent at both baseline and follow-up examinations.
Baseline examinations and measurements of the retinal vascular fractal dimension
In 1995, participants underwent an interview in which information on sex, age and diabetes duration was obtained. Further, data on BP, BMI, HbA1c, mean albumin excretion rate (AER) and VPT were assessed using previously described methods [15]. Baseline micro- and macroalbuminuria were defined as mean AERs of 20–200 μg/min and >200 μg/min, respectively, in at least two timed overnight urine samples. After pupillary dilation, colour retinal photographs were taken with 40°–60° retinal cameras, in accordance with European Diabetes Study Group (EURODIAB) recommendations [18]. Film slides were later digitalised using a DigitDia 5000 Filmscanner (Reflecta, Rottenburg, Germany). The disc–nasal field of the right eye was used for analyses of retinal vascular fractal dimensions. If the image of the right eye was ungradable, then the left eye was used for the analyses.
For the assessment of baseline DR and to rule out PDR, all photos were graded by the same certified grader using a modified Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol and allowing for non-standard photography in 1995, as previously described [14].
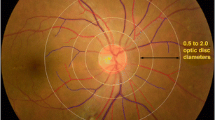
A semiautomated computer program was used to assess the retinal vascular fractal dimension (Singapore Institute Vessel Assessment-Fractal version 1.0, School of Computing, National University of Singapore, Singapore) by a standardised protocol. The optic disc was automatically detected by the software, all vessels were identified and those coursing through a zone 0.5–2.0 disc diameters from the disc margin were used for the analyses. The program provided skeletonised line tracing of the vasculature and, by comparing this to the original retinal colour image, any artefacts misidentified as vessels were removed by a certified grader. Examples of artefacts erroneously included in the line tracing are choroid vessels, pigment abnormalities and photograph halos. The retinal vascular fractal dimension was calculated from the refined line tracing by the box-counting method of the program, an established method for structures that are not perfectly self-similar, such as the retinal vasculature [19–21]. Figure 1 shows examples of retinal colour images and the corresponding refined line tracing for two of the participants in this study. The average time used for fractal analysis of each image in this study was 10 min.
Line tracings and zones. The retinal vascular fractal dimension is calculated from the line tracing in the marked zone 0.5–2.0 disc diameters (DD) from the disc margin; magnification ×1.84. The disc diameter is 1,800 μm in humans. (a) Cropped retinal image and line tracing of an eye in the tenth percentile with a fractal dimension of 1.3180 and a less complex vascular pattern. At follow-up, this participant had developed peripheral neuropathy, nephropathy and proliferative retinopathy. (b) Cropped retinal image and line tracing of an eye in the 90th percentile with a fractal dimension of 1.4678 and a more complex vascular pattern. At follow-up, this participant had neither peripheral neuropathy, nephropathy nor proliferative retinopathy
The image used for fractal analysis was further analysed using different image analysis software (IVAN, Department of Ophthalmology Visual Science, University of Wisconsin, Madison, WI, USA) to assess the retinal arteriolar and venular calibres. These were summarised using the ‘Big-6 formula’ into the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), respectively [22].
Outcome measures in 2011
Participants underwent examinations between 1 January and 1 November 2011. DPN was assessed with a hand-held biothesiometer (Bio-Medical Instrument, Newbury, OH, USA). Three measurements on the apex of the right first toe were made and the VPT was calculated as the mean of the last two. Participants with a VPT of >25 V were classified as having DPN.
At follow-up, all participants were asked to hand in spot urine samples unless they were menstruating or had ongoing infections; in those circumstances, they were asked to hand in samples at a later time. A spot urine albumin:creatinine ratio (ACR) was calculated from albumin and creatinine analyses performed on an Abbott Architect analyzer (Abbott, Deerfield, IL, USA) using immunoturbidimetric and enzymatic assays, respectively. Participants with macroalbuminuria (ACR ≥ 300 mg/g), with a history of kidney transplantation or who had received dialysis were all classified as having DN.
Participants had mydriatic colour retinal photographs taken by the same trained and certified operator using a 3D OCT-2000 Spectral Domain optical coherence tomography system (Topcon, Tokyo, Japan). Seven 45° fields were taken for each eye, in accordance with ETDRS standards [23]. A modified ETDRS scale was used for assessment of PDR, as previously described [14]. Participants with ETDRS levels of 61 or above in the eye included in the fractal analyses were classified as having PDR.
Statistical methods
Categorical data are presented as percentages, and continuous data are presented as means ± SD. We used the Mann–Whitney U test to assess differences between two groups, Cuzick’s test to identify trends among several groups and the Spearman rank correlation to test for associations between continuous variables. Multiple logistic regression analyses with backwards selection were performed to estimate ORs for incident DPN, DN and PDR. Models were adjusted for sex and baseline age, diabetes duration, HbA1c, systolic and diastolic BP, BMI, VPT, mean AER, level of retinopathy, CRAE, and CRVE.
All participants with a VPT of <25 V at baseline were considered at risk of incident DPN, and all participants without PDR at baseline were considered at risk of incident PDR. Patients with a baseline mean AER of >200 μg/min (indicating overt DN) were excluded from renal function analyses at follow-up.
For estimates of ORs, 95% CIs were considered statistically significant when they did not cross 1.0. Findings with a p value of <0.05 were considered statistically significant.
All statistical calculations were performed using Stata 11.1 (StataCorp, College Station, TX, USA).
Results
Study population characteristics
Of the 185 participants, fractal analysis was not possible in either eye of five patients (2.7%) because more than 25% of the vessels were untraceable within the predefined grid, in accordance with the standardised protocol. No significant differences in baseline characteristics between participants with and without gradable photos were found with respect to sex, baseline age, diabetes duration, HbA1c, BP, BMI, VPT, mean AER and level of retinopathy (data not shown). None of the five participants with non-gradable baseline photos for retinal vascular fractals had developed DPN, DN or PDR at follow-up.
A total of 180 patients with at least one gradable photo were included in the remaining analyses. For those participants, mean age and diabetes duration at baseline were 21.0 and 13.4 years, respectively, and 50.0% were males. The mean retinal vascular fractal dimension was 1.3817 (range 1.1932–1.5164).
Relationship between retinal vascular fractal dimension and clinical baseline characteristics
Table 1 shows that a lower retinal fractal dimension was significantly related to higher levels of both albuminuria and retinopathy at baseline, while there were no differences in fractal dimensions between males and females. When testing for rank correlations between retinal vascular fractal dimensions and the remaining baseline variables (i.e. age, diabetes duration, HbA1c, systolic and diastolic BP, BMI, VPT, CRAE and CRVE), no significant associations were found (data not shown).
Associations between retinal vascular calibres and incident DPN
DPN was evaluated at follow-up in 157 patients who had a VPT of <25 V at baseline in 1995 and gradable baseline photos. Of these, 10.8% (n = 17) had developed DPN in the intervening 16 years.
As seen in Table 2, a lower retinal vascular fractal dimension was significantly associated with incident DPN (OR 1.17 per 0.01 decrease in fractal dimension [95% CI 1.01, 1.36]) in the full multiple logistic regression model adjusted for sex and baseline age, diabetes duration, HbA1c, BP, BMI, VPT, mean AER, level of retinopathy, CRAE and CRVE. The same association was seen in models after adjusting for fewer baseline variables.
Associations of retinal vascular calibres and DN
A total of 163 participants with gradable baseline photos and no macroalbuminuria in 1995 handed in urine samples at follow-up. Of these, 8.0% (n = 13) had developed macroalbuminuria by 2011, had had a kidney transplant or had received dialysis by the time of follow-up.
A lower fractal dimension was significantly associated with the 16 year incidence of DN in all multiple logistic regression analyses carried out for this outcome, as seen in Table 2 (OR 1.40 per 0.01 decrease in fractal dimension in the full model [95% CI 1.10, 1.79]).
Associations between retinal vascular calibres and incident PDR
Of the 180 participants with gradable baseline photos, one had PDR in 1995 and was therefore excluded from this analysis. Of the 179 patients at risk, 27.9% (n = 50) progressed to PDR on the eye chosen for vessel analyses during the 16 year period. As shown in Table 2, the full multiple logistic regression found lower retinal vascular fractal dimension to be significantly associated with the 16 year incidence of PDR (OR 1.22 per 0.01 decrease in fractal dimension [95% CI 1.09, 1.37]), when adjusting for sex and baseline age, diabetes duration, HbA1c, BP, BMI, VPT, mean AER, level of retinopathy, CRAE and CRVE. The same association was seen in models that adjusted for fewer baseline variables.
Discussion
A lower retinal vascular fractal dimension was consistently associated with the 16 year incidence of DPN, DN and PDR in this prospective study of young Danish patients with type 1 diabetes. It was also associated with higher levels of albuminuria and retinopathy at baseline. These findings are consistent with existing cross-sectional studies [11, 12], but not with the single prospective report on incident retinopathy prediction [13]. However, for the latter study, the follow-up period was relatively short (mean 2.9 years) and all incident cases had mild retinopathy (ETDRS of ≤31). Therefore, no conclusions can be made on whether the retinal vascular fractal dimension can predict more severe levels of DR based on this study. Furthermore, the dynamic nature of DR creates misclassification issues because it is common for a patient to have minor signs of DR (ETDRS of ≤31) at one examination and none at the next, and vice versa.
The pathophysiological mechanisms underlying the findings in the present study are unclear, but endothelial dysfunction has previously been speculated to be the common link between microvascular complications in type 1 diabetes [24]. Several endothelium-generated vasoconstrictors are produced in abnormally high amounts in diabetes, the main one being endothelin-1 [25]. At the same time, impaired production of nitric oxide (a vasodilator) is observed in endothelial dysfunction [26]. Vasoconstriction may therefore contribute to the lower vessel density seen in our study. However, in early uncomplicated diabetes, both small and large blood vessels have been shown to dilate, not contract [27]. Nonetheless, dilation will increase blood flow and cause capillary hypertension, which will damage the endothelium over time. At this stage, impaired endothelium-dependent vasodilation can occur, thus reducing blood flow and leading to hypoxia. We therefore speculate that a lower fractal dimension of the retinal vasculature may be linked to vasoconstriction induced by endothelial dysfunction.
The autoregulatory response in the retinal vasculature is impaired in patients with diabetes and no retinopathy, and becomes progressively more impaired with increasing levels of retinopathy [28]. Thus, collateral formation in response to hypoxia may be altered, leading to a less complex vasculature than is normally required for optimal oxygenation. As the renal vasculature exhibits similar autoregulation, we speculate that this is likely to occur in the kidneys.
Genetics provides another perspective to these findings. It was previously suggested that subsets of patients with type 1 diabetes may have a genetic predisposition towards microvasculopathy because not all patients with high glucose levels develop complications [29]. Additionally, some patients develop complications despite having only slightly elevated glucose levels [30]. A less complex branching pattern of the microvasculature may provide a limited facility to form collaterals when metabolic demand exceeds the current oxygen supply, which will lead to hypoxia.
Studies have shown a reduction in the retinal fractal dimension with age [11, 31, 32]. If this represents a more general phenomenon for the systemic microvasculature, then some patients could be more prone to hypoxia-induced organ damage over time, simply as a result of suboptimal vascular geometry being already in place at diabetes onset.
In this study, a rather high incidence of PDR was seen. This was previously discussed in detail in another report [14] but, in brief, a high level of HbA1c at baseline (mean HbA1c 9.5% ± 1.6% [81 ± 17 mmol/mol]) in 1995) was believed to be the main cause.
Major strengths of this study are the population-based design, long follow-up time and use of quantitative methods for assessing retinal vascular fractal dimensions by standardised protocols. Furthermore, it was possible to adjust for a broad spectrum of baseline variables, including vessel diameter and the level of microvascular complications. Few patients (n = 5) had ungradable retinal photos and the remaining were all graded by the same certified and validated grader. Most participants had little or no vascular disease at baseline, which allowed a good estimate of the predictive value of the retinal fractal dimension to be made because progression to the end stage of any complication is associated with a major change in microvasculature.
A limitation is the relatively small number of participants and the low number of incident cases; nonetheless, our results were statistically significant. Intermediate measurements of the cohort would provide valuable information on the timeline of development of complications and correlating changes in retinal vasculature, but such data were not available.
In conclusion, our study has shown the retinal vascular fractal dimension to be a possible common preclinical biomarker of the three major complications seen in type 1 diabetes: DPN, DN and PDR. This indicates that diabetic microvascular complications share a common pathogenic pathway. Fractal analysis is therefore a potentially useful non-invasive tool for early risk stratification in diabetes regarding any microvascular complication. Fractal analysis of the retinal vasculature has previously demonstrated a high level of reproducibility [33]; however, the time to process each image needs to be shortened before its clinical use can be considered.
Abbreviations
- ACR:
-
Albumin:creatinine ratio
- AER:
-
Albumin excretion rate
- CRAE:
-
Central retinal arteriolar equivalent
- CRVE:
-
Central retinal venular equivalent
- DN:
-
Diabetic nephropathy
- DPN:
-
Diabetic peripheral neuropathy
- DR:
-
Diabetic retinopathy
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- PDR:
-
Proliferative diabetic retinopathy
- VPT:
-
Vibration perception threshold
References
Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ (2006) The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 55:1463–1469
Grauslund J, Green A, Sjolie AK (2009) Prevalence and 25 year incidence of proliferative retinopathy among Danish type 1 diabetic patients. Diabetologia 52:1829–1835
Grauslund J, Hodgson L, Kawasaki R, Green A, Sjolie AK, Wong TY (2009) Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia 52:2213–2217
Wong TY, Shankar A, Klein R, Klein BE (2004) Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes 53:179–184
Ding J, Cheung CY, Ikram MK et al (2012) Early retinal arteriolar changes and peripheral neuropathy in diabetes. Diabetes Care 35:1098–1104
Klein R, Klein BE, Moss SE, Wong TY, Sharrett AR (2006) Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology 113:1488–1498
Cheung CY, Lamoureux E, Ikram MK et al (2012) Retinal vascular geometry in Asian persons with diabetes and retinopathy. J Diabetes Sci Technol 6:595–605
Masters BR (2004) Fractal analysis of the vascular tree in the human retina. Annu Rev Biomed Eng 6:427–452
Cheung N, Donaghue KC, Liew G et al (2009) Quantitative assessment of early diabetic retinopathy using fractal analysis. Diabetes Care 32:106–110
Daxer A (1993) The fractal geometry of proliferative diabetic retinopathy: implications for the diagnosis and the process of retinal vasculogenesis. Curr Eye Res 12:1103–1109
Grauslund J, Green A, Kawasaki R, Hodgson L, Sjolie AK, Wong TY (2010) Retinal vascular fractals and microvascular and macrovascular complications in type 1 diabetes. Ophthalmology 117:1400–1405
Lim LS, Cheung CY, Sabanayagam C et al (2013) Structural changes in the retinal microvasculature and renal function. Invest Ophthalmol Vis Sci 54:2970–2976
Lim SW, Cheung N, Wang JJ et al (2009) Retinal vascular fractal dimension and risk of early diabetic retinopathy: a prospective study of children and adolescents with type 1 diabetes. Diabetes Care 32:2081–2083
Broe R, Rasmussen ML, Frydkjaer-Olsen U et al (2013) The 16-year incidence, progression and regression of diabetic retinopathy in a young population-based Danish cohort with type 1 diabetes mellitus: the Danish cohort of pediatric diabetes 1987 (DCPD1987). Acta Diabetol 51:413–420
Olsen BS, Johannesen J, Sjolie AK et al (1999) Metabolic control and prevalence of microvascular complications in young Danish patients with Type 1 diabetes mellitus. Danish Study Group of Diabetes in Childhood. Diabet Med 16:79–85
Olsen BS, Sjolie A, Hougaard P et al (2000) A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. J Diabetes Complicat 14:295–300
Mortensen HB, Marinelli K, Norgaard K et al (1990) A nation-wide cross-sectional study of urinary albumin excretion rate, arterial blood pressure and blood glucose control in Danish children with type 1 diabetes mellitus. Danish Study Group of Diabetes in Childhood. Diabet Med 7:887–897
Aldington SJ, Kohner EM, Meuer S, Klein R, Sjolie AK (1995) Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia 38:437–444
Macgillivray TJ, Patton N, Doubal FN, Graham C, Wardlaw JM (2007) Fractal analysis of the retinal vascular network in fundus images. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference, pp. 6456–6459
Stosic T, Stosic BD (2006) Multifractal analysis of human retinal vessels. IEEE Trans Med Imaging 25:1101–1107
Mainster MA (1990) The fractal properties of retinal vessels: embryological and clinical implications. Eye (Lond) 4(Pt 1):235–241
Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE (2003) Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 27:143–149
Anonymous (1985) Early Treatment Diabetic Retinopathy Study (ETDRS): manual of operations. U.S. Department of Commerce, National Technical Information Service
Zenere BM, Arcaro G, Saggiani F, Rossi L, Muggeo M, Lechi A (1995) Noninvasive detection of functional alterations of the arterial wall in IDDM patients with and without microalbuminuria. Diabetes Care 18:975–982
Tesfamariam B, Jakubowski JA, Cohen RA (1989) Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol 257:H1327–H1333
Harrison DG (1997) Endothelial function and oxidant stress. Clin Cardiol 20, II-11–17
Vervoort G, Wetzels JF, Lutterman JA, van Doorn LG, Berden JH, Smits P (1999) Elevated skeletal muscle blood flow in noncomplicated type 1 diabetes mellitus: role of nitric oxide and sympathetic tone. Hypertension 34:1080–1085
Grunwald JE, Riva CE, Brucker AJ, Sinclair SH, Petrig BL (1984) Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology 91:1447–1452
The Diabetes Control and Complications Trial Research Group (1995) The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44:968–983
Reichard P (1995) Are there any glycemic thresholds for the serious microvascular diabetic complications? J Diabetes Complicat 9:25–30
Liew G, Wang JJ, Cheung N et al (2008) The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology 115:1951–1956
Azemin MZ, Kumar DK, Wong TY et al (2012) Age-related rarefaction in the fractal dimension of retinal vessel. Neurobiol Aging 33(194):e191–e194
Cosatto VF, Liew G, Rochtchina E et al (2010) Retinal vascular fractal dimension measurement and its influence from imaging variation: results of two segmentation methods. Curr Eye Res 35:850–856
Funding
This work was supported by Fight for Sight, Denmark, the Synoptik Foundation, Medivit Aps, the Gangsted Foundation, the Foundation of Karen Svankjaer Yde, a Lykfeldts grant, the A.P. Moeller Foundation for the Advancement of Medical Science, the Region of Southern Denmark and the University of Southern Denmark.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
RB, JG, BSO and HBM contributed to the study concept and design. RB, MLR, UF-O, BSO and HBM contributed to data acquisition. Data analyses were performed by RB, who also wrote the initial draft of the paper. MLR, UF-O, BSO, HBM, TP and JG were involved in the interpretation of data and revised the paper critically for intellectual content. RB is the guarantor of this work and, as such, had full access to all study data and takes full responsibility for the integrity of the data, the accuracy of the data analysis, and for the decision to submit for publication. All authors approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Broe, R., Rasmussen, M.L., Frydkjaer-Olsen, U. et al. Retinal vascular fractals predict long-term microvascular complications in type 1 diabetes mellitus: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetologia 57, 2215–2221 (2014). https://doi.org/10.1007/s00125-014-3317-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3317-6