Article Text
Abstract
Objective A simulation model was constructed to assess long-term outcomes of proactively treating severe non-proliferative diabetic retinopathy (NPDR) with anti-vascular endothelial growth factor (anti-VEGF) therapy versus delaying treatment until PDR develops.
Methods and analysis Simulated patients were generated using a retrospective real-world cohort of treatment-naive patients identified in an electronic medical records database (IBM Explorys) between 2011 and 2017. Impact of anti-VEGF treatment was derived from clinical trial data for intravitreal aflibercept (PANORAMA) and ranibizumab (RISE/RIDE), averaged by weighted US market share. Real-world risk of PDR progression was modelled using Cox multivariable regression. The Monte Carlo simulation model examined rates of progression to PDR and sustained blindness (visual acuity <20/200) for 2 million patients scaled to US NPDR disease prevalence. Simulated progression rates from severe NPDR to PDR over 5 years and blindness rates over 10 years were compared for delayed versus early-treatment patients.
Results Real-world data from 77 454 patients with mild-to-severe NPDR simulated 2 million NPDR patients, of which 86 680 had severe NPDR. Early treatment of severe NPDR with anti-VEGF therapy led to a 51.7% relative risk reduction in PDR events over 5 years (15 704 early vs 32 488 delayed), with a 19.4% absolute risk reduction (18.1% vs 37.5%). Sustained blindness rates at 10 years were 4.4% for delayed and 1.9% for early treatment of severe NPDR.
Conclusion The model suggests treating severe NPDR early with anti-VEGF therapy, rather than delaying treatment until PDR develops, could significantly reduce PDR incidence over 5 years and sustained blindness over 10 years.
- macula
- pharmacology
- treatment medical
- vision
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is a lack of long-term clinical trial data and real-world evidence on the impact of anti-vascular endothelial growth factor (anti-VEGF) treatment at the severe non-proliferative diabetic retinopathy (NPDR) stage on progression to PDR and long-term visual outcomes in this population.
WHAT THIS STUDY ADDS
Monte Carlo simulation model results suggest that anti-VEGF treatment at the severe NPDR stage could significantly reduce incidence of PDR over 5 years and sustained blindness over 10 years.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides an estimation of the impact of treatment at the severe NPDR stage in a large simulated NPDR population derived from a real-world database.
Introduction
Diabetic retinopathy (DR) is a leading cause of vision loss and blindness in people aged 20–65 years.1 2 With progression from non-proliferative DR (NPDR) to PDR, risks of ocular complications increase, including retinal detachment, vitreous haemorrhage and diabetic macular oedema (DMO), which can lead to severe vision loss and blindness.2–4
Greater DR severity is associated with faster PDR progression5 6 and sustained blindness when untreated.7 In the Early Treatment Diabetic Retinopathy Study, progression rates from NPDR to PDR in untreated eyes increased with greater baseline DR severity.5 Recent US clinical practice data showed 46.8% of untreated eyes with severe NPDR progressed to PDR within 4 years. The probability of sustained blindness over 2 years increased with DR severity; eyes with severe NPDR and PDR at diagnosis were 3.6 and 4.0 times more likely, respectively, to develop sustained blindness than those with mild NPDR.7 Anti-vascular endothelial growth factor (anti-VEGF) treatment slows progression to PDR, development of vision-related complications and centre-involved DMO (CI-DMO) with vision loss in patients with NPDR.8–10 For patients with moderately severe or severe NPDR at baseline, intravitreal aflibercept improved Diabetic Retinopathy Severity Scale (DRSS) score and reduced risk of developing a vision-threatening complication (PDR/anterior segment neovascularisation) or CI-DMO over 100 weeks versus sham.8 In patients with moderately severe to severe NPDR, lower DR worsening rates were observed following ranibizumab treatment versus sham through month 36.9 In Protocol W, intravitreal aflibercept-treated patients with moderate-to-severe NPDR had lower rates of PDR or CI-DMO with vision loss through 2 years versus sham.10
Long-term clinical trial data and real-world evidence examining the impact of early anti-VEGF treatment on progression of severe NPDR to PDR and visual outcomes in this population are lacking. We developed a Monte Carlo simulation (MCS) model to assess the impact of initiating anti-VEGF therapy when mild or moderate NPDR progresses to severe NPDR (DRSS 47–53)—rather than delaying until PDR develops—on the rate of PDR progression and incidence of associated blindness.
Materials and methods
A real-world cohort of treatment-naive patients with mild-to-severe NPDR was identified using IBM Explorys, a database of deidentified, longitudinal patient-level data from over 53 million patients, from electronic health records (EHR) and billing sources within participating Integrated Delivery Networks, Clinically Integrated Networks and Care Collaborative Networks. This cohort was used to estimate real-world rates of PDR progression, and provided patient profiles to generate a larger population of simulated patients, weighted to reflect NPDR prevalence in the US, used in an MCS model (online supplemental eMethods). This model followed a simulated cohort of patients to evaluate PDR progression rates and subsequent development of sustained blindness through 2 scenarios: early treatment during severe NPDR or delayed treatment when PDR developed.
Supplemental material
Identification of a real-world cohort of patients with NPDR
The real-world cohort comprised patients aged ≥18 years with an incident NPDR diagnosis (mild, moderate, severe or unspecified) between 2011 and 2017 (diagnosis date referred to as index date). Briefly, patients had not received anti-VEGF, pan-retinal photocoagulation or steroid medications 1 year before index date; had no vitreous haemorrhage, retinal detachment, retinal vein occlusion or neovascularisation in the year before or week after NPDR diagnosis; and had no PDR diagnosis within 1 week after index date (online supplemental eTable 1).
NPDR diagnosis was determined based on Systematized Nomenclature of Medicine codes (online supplemental eMethods).
Simulation model
The real-world cohort was developed using MySQL Workbench V.8.0. Cox proportional hazards and MCS models were coded/analysed using Python programming language in Spyder V.3.6. A simulated cohort of 2 million patients with mild-to-severe NPDR was generated by random sampling with replacement (bootstrapping) from the real-world cohort. The greater size of the simulation cohort relative to the database cohort is consistent with principles of MCS, with bootstrapping allowing for multiple replications per individual.11 With a sample size of 2 million, diagnostic plots representing key summary results from simulation versus sample size were stable (variation <0.1% with successive runs) and insensitive to further cohort size increases. Sampling weights were used in bootstrapping to account for quantifiable differences in characteristics of the real-world cohort relative to the overall US NPDR population (online supplemental eMethods).
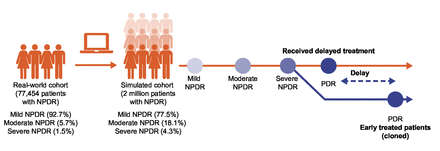
In the MCS model, to ensure the only difference between groups was treatment effect, patients developing severe NPDR were ‘cloned’ into two identical subcohorts: early anti-VEGF treatment at the severe NPDR stage and delayed treatment until PDR development (figure 1). Patients in the simulated cohort followed their own probabilistic path through NPDR progression to PDR based on their risk profile applied to the Cox regression risk equation. Anti-VEGF treatment impact was derived from efficacy estimates of clinical trial data for intravitreal aflibercept 2 mg dosed every 8 weeks after 5 monthly doses (2q8; PANORAMA)8 and intravitreal ranibizumab 0.3 mg dosed monthly (post-hoc analysis of RISE/RIDE)9 (online supplemental eTable 5), in both separate and combined scenarios. Only year 1 data were used, as the 2q8 group in PANORAMA received treatment as needed in year 2. Variability in risk of PDR progression was estimated in the combined treatment scenario using bootstrapping (online supplemental eMethods).
Flow of the simulation model. Circles represent first respective diagnosis. Patients receiving delayed treatment were followed from the index diagnosis through all stages of NPDR progression up to the first PDR event. NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Outcomes
The simulated rate of PDR progression was assessed in untreated patients with mild, moderate or severe NPDR over 5 years. The impact of early anti-VEGF treatment on rates of PDR progression was assessed for patients with severe NPDR projected over 5 years. Rates of sustained blindness with PDR projected over 10 years were assessed in patients who received anti-VEGF for severe NPDR (early treatment) versus those who were treated after PDR developed (delayed treatment). Sustained blindness was defined as visual acuity (VA) of ≤20/200 in the study eye at 2 visits ≥3 months apart, with no improvement >20/100 since the first ≤20/200 reading.
Statistical analysis
A Cox proportional hazard model was used to estimate individual patient risk of PDR progression, calibrated to match with average cohort risks obtained by Kaplan-Meier analysis (online supplemental eTable 6 and eMethods). Estimated rates of PDR progression were based on specific patient demographic and clinical characteristics. Patients’ annual risks could vary over the projected 5-year model if impacted by changes in their age group or NPDR severity.
The EHR database cannot fully capture progression through mild, moderate and severe NPDR. Therefore, differences in PDR progression hazards were used to estimate disease progression rates for untreated patients with mild-to-moderate and moderate-to-severe NPDR in the real-world cohort. Rates of disease progression post-treatment were linearly projected to 5 years for patients with severe NPDR using year 1 data for the intravitreal aflibercept 2q8 arm of the 2-year PANORAMA trial.8
Sustained blindness rates over 10 years were estimated based on rates reported by Wykoff et al for PDR7 and projected based on PDR event rates in early and delayed treatment groups over 5 years (with treatment effect from clinical trials not applied beyond 5 years). Alternative estimates of blindness were based on linear projections of the Diabetic Retinopathy Study (DRS) (VA <5/200 at ≥2 consecutive 4-month follow-up visits).12
Model assumptions
Patients would not be treated earlier than the severe NPDR stage, not accounting for other reasons for anti-VEGF treatment. The real-world population was scaled to reflect quantifiable differences with the US population using IBM Market Scan 2018 data, however, demographics and clinical characteristics are likely to change over 10 years. Patients were assumed to receive the same dosing regimen as the clinical trials, although in real life there may be altered dosing, missed treatments, patients lost to follow-up and treatment interruptions or discontinuation. The US market share data for anti-VEGF agents was assumed valid over 10 years (confounding factors may include new treatments and generics). Data from the clinical trials (PDR progression risk and treatment impact) at year 1 were assumed to be applicable over 1–5 years. Results from the trials were assumed to be applicable to the simulated cohort, despite inclusion/exclusion criteria differing from the real-world population, the basis of the simulated population. For example, the real-world cohort included patients with DMO, while PANORAMA did not. Since moderately severe NPDR is not captured in the IBM Explorys database, severe NPDR would be a combination of moderately severe and severe NPDR. Patients included in the Wykoff et al study7 used for blindness risk estimates were treated appropriately. Finally, the model assumed patients developed PDR before sustained blindness.
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of our research in any way.
Results
Of 141 451 patients with NPDR identified from IBM Explorys between 2011 and 2017, 77 454 were included in the real-world cohort (online supplemental eFigure 1). Most were classified as mild or unspecified NPDR (92.7%), followed by moderate (5.7%) and severe (1.5%) NPDR (online supplemental eTable 7).
Compared with the real-world cohort, the simulated cohort had a higher proportion of patients aged 65–74 years (32.4%), with DMO (23.0%), and with moderate (18.1%) and severe (4.3%) NPDR.
Risk factors for PDR events
Risk factors for PDR included greater baseline NPDR severity, DMO, certain comorbidities (eg, diabetic nephropathy, diabetic neuropathy), medications (eg, beta blockers) and laboratory results (eg, glycated haemoglobin, insulin) (online supplemental eTable 6). Diabetic nephropathy at baseline was associated with a 23% increase in hazard of progressing to PDR versus no diabetic nephropathy. Age ≥55 years, male sex, better glucose management, lower rates of hypertriglyceridaemia, hypertension, cerebrovascular accident or amputation were associated with decreased risks of PDR events.
Rate of disease progression in the real-world cohort
In the real-world cohort, there was a steady increase in estimated cumulative disease progression risk in untreated patients from mild-to-moderate (12.4%) and moderate-to-severe NPDR (18.8%) at 5 years (online supplemental eFigure 2A).
Based on PANORAMA year 1 data, patients with moderately severe to severe NPDR had a lower estimated cumulative risk of disease progression when treated with intravitreal aflibercept (9.6%) versus no treatment (18.8%) projected at 5 years (online supplemental eFigure 2B).
Rate of progression to PDR in the simulated cohort
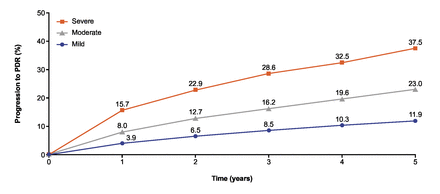
In the simulated cohort, PDR progression rates projected over 5 years were higher in untreated patients with severe NPDR (37.5%) versus moderate (23.0%) or mild (11.9%) NPDR (figure 2).
Risk of progression to PDR in untreated simulated patients by NPDR severity over 5 years. NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
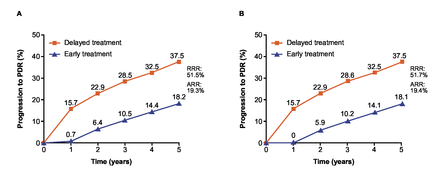
Based on PANORAMA year 1 results, early intravitreal aflibercept treatment reduced PDR progression risk projected over 5 years in simulated patients with severe NPDR by 51.5% (18.2% vs 37.5% with delayed treatment; absolute risk reduction 19.3%) (figure 3A). Comparable results were obtained from a post hoc analysis of RISE/RIDE year 1 data projected over 5 years, in which early treatment of severe NPDR with intravitreal ranibizumab resulted in a 51.7% reduction in PDR event risk versus delayed treatment (figure 3B).
Risk of progression to PDR in delayed versus early treated simulated patients with severe NPDR projected over 5 years based on year 1 data in the (A) PANORAMA and (B) RISE/RIDE trials. ARR, absolute risk reduction; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RRR, relative risk reduction.
Impact of early anti-VEGF treatment on the rate of progression to PDR
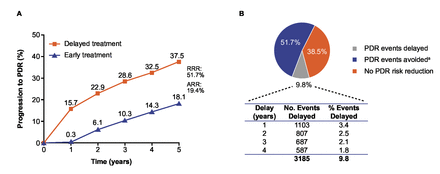
In the composite treatment scenario (projected over 5 years based on PANORAMA and RISE/RIDE year 1 data), 16 784 (51.7%) PDR events were avoided over 5 years with early anti-VEGF treatment (15 704 PDR events vs 32 488 with delayed treatment) in patients with severe NPDR, a 19.4% absolute risk reduction (figure 4A, online supplemental eTable 8). Additionally, 3185 (9.8%) PDR events were delayed over 5 years with early versus delayed treatment (figure 4B). When stratified by diabetes type, a higher proportion of PDR events were avoided in early treated patients with type 2 diabetes (10.5%; 28 978 of 275 349 events avoided) versus type 1 diabetes (7.8%; 1930 of 24 731 events avoided), an absolute risk reduction of 1.6% versus 1.4%, respectively (online supplemental eTable 8).
Composite treatment scenario (based on year 1 data from PANORAMA and RISE/RIDE trials) of patients with severe NPDR: impact of early anti-VEGF treatment on (A) risk of progression to PDR and (B) PDR events projected over 5 years. a‘PDR events avoided’ refers to percentage of patients who did not experience a PDR event with early treatment compared with what would be expected with delayed treatment. ARR, absolute risk reduction; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RRR, relative risk reduction; VEGF, vascular endothelial growth factor.
Rate of sustained blindness
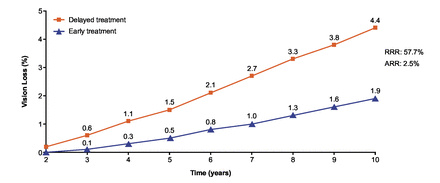
In simulated patients with severe NPDR, sustained blindness events with PDR were reduced by 57.7% projected over 10 years with early versus delayed anti-VEGF treatment (1.9% vs 4.4%, respectively), a 2.5% absolute risk reduction (figure 5).
Impact of anti-VEGF treatment initiation (early vs delayed) on the rate of sustained blindnessa over 10 years. aSustained blindness defined as ≥2 VA readings of 20/200 or worse ≥3 months apart, and no improvement beyond 20/100 after the first 20/200 reading. First data point simulated 2 years after model start. Projections of sustained blindness rates based on PDR events estimated by Wykoff et al.7 ARR, absolute risk reduction; PDR, proliferative diabetic retinopathy; RRR, relative risk reduction; VA, visual acuity; VEGF, vascular endothelial growth factor.
Sensitivity analysis
A sensitivity analysis, conducted to examine variability in risk estimates for the composite treatment scenario (using ±5% variability), found that PDR progression risk projected over 5 years was reduced to half in early treated patients with severe NPDR versus delayed treatment. Relative risk reductions ranged from 31.1% to 66.5% and absolute risk reductions from 10.4% to 28.2% (online supplemental eFigure 3). The 95% CIs for early and delayed treatment groups did not overlap, suggesting that, after accounting for variability, PDR progression was significantly reduced with early anti-VEGF treatment.
An additional sensitivity analysis was performed using alternative estimates for blindness (VA <5/200) based on the DRS study.12 Rerunning the model with this input, the projected incidence of blindness with early treatment (4.2%) versus delayed treatment (9.9%) in patients with severe NPDR decreased 58.0% over 10 years (online supplemental eFigure 4).
Discussion
Long-term clinical trial data and real-world evidence are lacking regarding effects of early anti-VEGF treatment as a function of baseline NPDR stage on PDR progression and long-term visual outcomes in this population. This study aimed to improve current understandings of impacts of early treatment in a large simulated mild, moderate and severe NPDR population derived from a real-world EHR database and adjusted to represent the US NPDR population. This provided a more generalisable characterisation of impacts and clinical value of early anti-VEGF treatment beyond what is captured in clinical trials.
In this simulation model, early treated patients with severe NPDR had a significantly lower risk of PDR progression and blindness than patients receiving delayed treatment. PDR progression rates projected over 5 years increased with NPDR severity and were higher in patients with severe (37.5%) versus mild (11.9%) or moderate (23.0%) NPDR. Left untreated, the projected 4-year risk of PDR progression from severe NPDR in this study (32.5%) was lower than that observed in a recent retrospective study (46.8%), possibly due to patient population differences (eg, fewer patients with type 1 diabetes and moderate or severe NPDR).
Furthermore, a sensitivity analysis using a ±5% variability in risk estimates confirmed a 37.8%–18.4% reduction in the projected 5-year risk of PDR development with early treatment in patients with severe NPDR. An additional 9.8% of PDR events were delayed 1–4 years within the same period. A 0.5% variation in mean risk estimates was acceptable due to stochasticity in the model. The alternative projections for blindness, evaluated using DRS estimates,12 confirmed a considerable reduction in blindness risk with early treatment of patients with severe NPDR projected over 10 years.
Early treatment of patients with severe NPDR projected over 10 years was associated with a ~58% reduction in sustained blindness. The estimated 10-year sustained blindness risk with PDR was 4.4% with delayed treatment, decreasing to 1.9% with early anti-VEGF treatment.
Findings from this study are broadly consistent with prior studies in DR progression that highlight the importance of close monitoring and early treatment at the NPDR stage to reduce PDR progression.6 8 9 One recent retrospective analysis found the risk of progression to severe NPDR or PDR within 5 years of diagnosis was approximately 3-fold greater in treatment-naive patients with moderate (17.6%) versus mild (5.8%) NPDR, highlighting the importance of closely monitoring these patients.6 In Protocol W, the 2-year cumulative rate of developing PDR among eyes with moderate-to-severe NPDR was reduced with intravitreal aflibercept treatment versus sham (13.5% vs 33.2%, respectively).10
The estimated impact of early anti-VEGF intervention observed in this model may help fill the data gap in the existing evidence from controlled clinical trials that report data up to 2 years.8–10 Since each sampled patient traced a unique probabilistic path and treatment response over the model time horizon, the study outcomes may exaggerate treatment effect estimates possibly observed in real-world settings. The MCS model is widely used as a standard for modelling disease progression and understanding optimal treatment strategies and practice patterns in other therapeutic areas.13–16 Intrinsic features of flexibility and scalability of this model allow for future adaptations to account for real-world challenges like non-adherence or payer restrictions. Model findings could stimulate clinical discussions about benefits of early anti-VEGF interventions for severe NPDR and potentially be of value to inform clinical practice patterns.
Use of an EHR database for developing the study cohort may introduce specific biases as data related to NPDR severity, clinical management (eg, treatment adherence, anti-VEGF injection frequency) and disease progression may not be fully captured. Adjusted sampling methodology helped ensure summary characteristics of the simulation cohort were similar to the representative US NPDR population. Although this study accounted for quantifiable biases based on age, sex and clinical conditions, not all biases can be measured or corrected. For example, the population-adjusted cohort had a higher proportion of patients with DMO versus the real-world cohort (23% vs 1%, respectively), likely due to exclusion of treated patients at baseline in the real-world cohort. Differences in patient population and clinical management of NPDR may result in lower treatment efficacy in real life compared with results observed in PANORAMA and RISE/RIDE.
Other limitations include a high proportion of patients with mild or unspecified NPDR, likely due to incomplete EHR entries in the database. Since intermediate NPDR stages were not always captured, progression rates from mild-to-moderate and moderate-to-severe NPDR were estimated based on PDR progression hazard differences. For PANORAMA, NPDR progression rates post-treatment were linearly projected to 5 years using year 1 data as 2q8 dosing during year 2 was as needed.8 Since moderately severe NPDR is not captured in the IBM Explorys database, severe NPDR was assumed a combination of moderately severe and severe NPDR. Yet, in treatment scenarios, anti-VEGF efficacy in patients with moderately severe to severe NPDR was applied to the severe NPDR cohort. Risk of PDR progression may be underestimated, as only PDR events during follow-up periods were tracked. Due to lack of VA data in the real-world cohort, vision loss rates were estimated using projections from previously published literature.7 However, the study by Wykoff et al did not account for treatment of diabetes or DR in their study7; we assumed patients included were treated appropriately. The MCS model relied on data from PANORAMA and RISE/RIDE, and did not account for patient compliance and clinical management of NPDR in a real-world setting.
The MCS model suggests early treatment of severe NPDR with intravitreal anti-VEGF therapy, rather than delaying until PDR develops, could significantly decrease PDR occurrence over 5 years and reduce incidence of sustained blindness with PDR over 10 years. This simulation may provide a reasonable alternative for estimating the impact of initiating treatment at the severe NPDR stage in the absence of long-term clinical trial data.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
This study did not enrol human participants and used deidentified patient data; institutional review board approval or patient informed consent was not required.
Acknowledgments
Medical writing support under guidance of the authors was provided by Rhutika Dessai, MSc, and Rob Campbell, PhD, and editing support was provided by Joe Alling, BSc, all of Core, London, UK, in accordance with Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460), and funded by Regeneron Pharmaceuticals, Inc.
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Presented at The results of this study were presented in parts at the 44th Macula Society 2021 Virtual Annual Meeting, 6–7 February 2021; the Association for Research in Vision and Ophthalmology 2021 Annual Meeting, 1–7 May 2021, Rockville, Maryland; and the American Academy of Ophthalmology 2021 Annual Meeting, 12–15 November 2021, New Orleans, Louisiana.
Contributors All authors contributed to the concept, design, data collection, interpretation, analysis and drafting of the manuscript. QDN and SS act as guarantors, and take full responsibility for the work, had access to the data, and controlled the decision to publish.
Funding The study was funded by Regeneron Pharmaceuticals, Inc, Tarrytown, New York. The sponsor participated in the design and conduct of the study, analysis of the data and preparation of this manuscript.
Competing interests QDN: Scientific advisory boards for Bausch + Lomb, Genentech, Regeneron Pharmaceuticals, Inc, Santen and Unity. AAM: Consultant to Allergan, Genentech/Roche, Graybug, Novartis, Ocular Therapeutix, Regeneron Pharmaceuticals, Inc, Pr3vent, Waldo, Valitor and Regenxbio; contracted research with Genentech/Roche, Novartis and Regeneron Pharmaceuticals, Inc; and ownership interest in Ocular Therapeutix, PLACID0, Waldo and Pr3vent. JIL: Consultant to Allergan, Aura Biosciences, Cognition, Eyenuk, Genentech, Luxa, Novartis, Opthea, Quark, Santen, Unity, and Viridian; research funds from Aldeyra Therapeutics, Chengdu, Genentech, Regeneron Pharmaceuticals, Inc, NGM, Stealth and Graybug; and honoraria from Iveric Bio. EP: Consultant to Regeneron Pharmaceuticals, Inc. AC: Consultant to Regeneron Pharmaceuticals, Inc. RR: Employee of and stockholder in Regeneron Pharmaceuticals, Inc. SS: Employee of and stockholder in Regeneron Pharmaceuticals, Inc.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.