Article Text
Abstract
Background We conducted a study to investigate the relationship between optic nerve vertical cup-to-disc ratio (VCDR), body and ocular parameters, and brain lesions in middle-aged and above Japanese subjects, because although various risk factors for glaucoma have been previously characterised, it is theorised that there are unidentified neurological components.
Methods In this population-based, age/gender-stratified, cross-sectional study that involved 2239 Japanese subjects (1127 men and 1112 women) aged 40 years and older (mean age: 59.3±11.7 years) living in the central geographical region of Japan who participated in the National Institute of Longevity Sciences–Longitudinal Study of Aging between 2002 and 2004, 4327 eyes and 2239 obtained MRIs of the head were evaluated. Multivariate mixed model and trend analyses were also performed.
Results No significant relationship between VCDR and brain lesions, other than basal ganglia lesions, was found. VCDR significantly increased with the high grade of basal ganglia infarct lesions (p=0.0193) and high intraocular pressure (p<0.0001) after adjustment for influential factors using a multivariate mixed model. A significant positive linear trend was observed between the predicted VCDR and the degrees of the basal ganglia lesions (p value trend=0.0096).
Conclusion Our findings suggest that in subjects with higher grades of basal ganglia lesions, strict attention should be paid to elevated VCDR; however, further studies are needed to support/confirm our results.
- Intraocular pressure
- Epidemiology
- Optic Nerve
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although various risk factors for elevated vertical cup-to-disc ratio have been previously characterised, there were no identified neurological components.
WHAT THIS STUDY ADDS
Vertical cup-to-disc ratio significantly increases with a high degree of basal ganglia lesions after being adjusted for age, gender, optic disc area, systolic blood pressure, diastolic blood pressure, body mass index, central corneal thickness and a history of cataract surgery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings suggest that in subjects with higher grades of basal ganglia lesions, strict attention should be paid to elevated vertical cup-to-disc ratio. Since the detailed mechanism remains unclear, further studies are expected and needed to elucidate how basal ganglia lesions affect optic nerve disc cupping, as well as whether they are a cofactor or causal factor of elevated vertical cup-to-disc ratio.
Introduction
Glaucoma is a major public health problem and the main cause of irreversible blindness.1 A previous study reported that due to the dramatic increase in ageing populations worldwide, the incidence of primary open-angle glaucoma (POAG) is estimated to rise to 111.8 million in 2040.2 Glaucoma is a progressive optic neuropathy characterised by both ocular morphological changes, such as cupping of the optic nerve disc and loss of retinal ganglion cells (RGCs), and development of glaucomatous visual field defects that correspond with the loss of the area of the optic neuronal rim. Several theories for the pathogenesis of glaucomatous optic neuropathy have previously been described.3 4 Those theories are derived from the risk factors for POAG that have thus far been investigated; that is, high intraocular pressure (IOP),5 advancing age,6 gender,7 family history, myopia, ethnicity,1 high systolic blood pressure, low diastolic blood pressure,8 diabetes mellitus,9 low body mass index (BMI) and low cerebrospinal fluid (CSF) pressure.10
The vertical cup-to-disc ratio (VCDR) is a highly sensitive and important indicator for diagnosis, detection of structural changes and monitoring in the early to moderate stages of glaucoma11 because the visual field defects in glaucoma are often detected only after 20%–40% of the axons are lost.12
IOP, which is believed to be the most significant factor for glaucoma,13 reportedly has a positive correlation with central corneal thickness (CCT).14 It has also been reported that BMI and systolic blood pressure are directly correlated with IOP.15 In addition, positive associations between BMI and blood pressure have been documented in several ethnicities.16 Several reports suggest that a high BMI protects against glaucoma, whereas a low BMI is a possible risk factor.10 Moreover, the above-described risk factors for glaucoma are intricately inter-related.
Even though a low BMI related to a low IOP is considered to be one of the risk factors for glaucoma, a high IOP is believed to be the most significant risk factor. With this discrepancy in mind, we theorised that other factors may also be responsible for glaucomatous optic nerve damage.
The purpose of this present study was to investigate the relationship between VCDR and brain lesions. We theorise that a relationship may exist between eye disease and brain lesions, since the optic nerve and neuronal retinal layer are derived embryologically from the same precursor as brain tissue.17
Materials and methods
Study design and participants
Study participants were selected from the third wave of examinations (May 2002–May 2004) of the National Institute for Longevity Sciences–Longitudinal Study of Aging (NILS-LSA). NILS-LSA is a longitudinal, dynamic cohort study that includes medical, physiological, nutritional and psychological examinations. A total of 2378 male and female subjects were randomly selected from Obu and Higashiura, cities located in the central geographical region of Japan. It should be noted that no restrictions were applied in regard to the inclusion criteria. After excluding the participants with missing data (n=137), we selected 2241 right eyes and head MRIs (1138 men and 1103 women; mean age 59.1±11.6 years) for stepwise multiple regression analysis, and 4327 eyes and 2239 head MRIs (1127 men and 1112 women; mean age, 59.3±11.7 years) with no MRI-incompatible metallic implants for final multivariate analysis. Patients or the public were involved in the design, or conduct, or reporting, or dissemination plans of our research.
Brain MRI acquisition
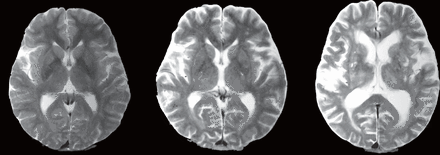
Brain MRI was performed on the participants using a 1.5 T scanner (Visart 1.5 T MRI System; Toshiba Corporation, Tokyo, Japan). The MRIs were visually inspected by neurological specialists, and images with motion artefacts were excluded from this study. The presence and the degree of brain infarct lesions (due to lacunar infarction, brain embolism, and/or brain thromboembolism) in various regions, including the basal ganglia, thalamus, cerebellar white matter, cerebellar grey matter, cerebral white matter, cerebral grey matter, paralateral ventricles, mesencephalon and pons, as well as any deeper areas affected by lacunar infarctions, were graded as follows: grade 0 (no lesions), grade I (unilateral lesions) and grade II (bilateral lesions) (figure 1), or grade 0 (no lesion) and grade I (lesion present). Due to the resolution limit of 1.5 T MRI, we labelled the faint lesions centred around the putamen overlapping adjacent borders and/or lesions in the globus pallidus, internal capsule and caudate nucleus as basal ganglia lesions.
Representative MRIs of basal ganglia lesions. Basal ganglia lesions were graded as (left) grade 0 (no lesions), (middle) grade I (unilateral lesions) or (right) grade II (bilateral lesions). The images have been altered to mask the identity of the patients.
Ocular parameters
CCT findings were obtained via non-contact specular microscopy (SP-2000; Topcon Corporation, Tokyo, Japan), and VCDR and optic disc area were assessed via Heidelberg retinal tomography (HRT) (HRT II; Heidelberg Engineering, Heidelberg, Germany). In each eye, IOP was measured by use of a non-contact tonometer (NT-3000; NIDEK Co, Gamagori, Japan).
Body parameters
Blood pressure was measured at the upper right arm while the subject was in the sitting position. BMI was calculated by dividing the square of the subject’s height (in square metres) from their weight (in kilograms). The acquisition of the ocular parameters, the body parameters and the brain MRI was all carried out on the same day.
Statistical analysis
The data were processed and analysed using the Statistical Analysis System (SAS V.9.3, SAS Institute). We used four age groups (subjects in their 40s, 50s, 60s and 70s) from the fourth decade group (40–49 years of age) to the seventh decade group (70 or more years of age). Only the right eye data of each subject were evaluated for stepwise multiple regression analysis. VCDR was classified by cup-to-disc ratio (CDR) classes (CD1, VCDR <0.3; CD2, 0.3≤VCDR<0.6; CD3, 0.6≤VCDR<0.7; CD4, 0.7≤VCDR<0.9; CD5, 0.9≤VCDR).18 We evaluated the associations between VCDR and brain infarct lesions in various regions, including basal ganglia (globus pallidus, internal capsule and caudate nucleus), thalamus, cerebellar white matter, cerebellar grey matter, cerebral white matter, cerebral grey matter, paralateral ventricles, mesencephalon and pons, as well as any deeper areas affected by lacunar infarctions, using stepwise multiple regression analysis after adjusting for age groups and gender. In addition, the stratified table including statistically significant factors for stepwise multiple regression analysis and age groups was analysed using the Cochran-Mantel-Haenszel statistic.
Next, VCDR was analysed using a mixed-effect model, a type of statistical analysis used for repeated measurements and binomial values of a single individual. Data from both eyes of each subject were evaluated in this analysis due to the uncertainty of being able to determine whether a single eye with elevated VCDR would be associated with ipsilateral, contralateral or bilateral (asymmetrical or symmetrical) lesions of the brain, and because glaucoma can develop unilaterally or bilaterally. The analysis was applied using the SAS procedure PROC MIXED with RANDOM INTERCEPT/SUB=ID. Explanatory variables included IOP, CCT, BMI, the grade of basal ganglia lesions and other statistically significant factors. The data were analysed after being adjusted for age, gender, optic disc area, systolic blood pressure, diastolic blood pressure and a history of cataract surgery. In addition, the predicted VCDR was calculated and analysed using the ESTIMATE and CONTRAST command as statistically significant factors were varied at different degrees (0, I or II). The level of significance in all analyses was set at p<0.05.
Results
The characteristics of the participants who completed head MRIs are presented in table 1.
Ophthalmic characteristics and grades of the brain lesions that participated in head MRI
The mean IOP, CCT, VCDR and BMI were 12.72±2.52 mm Hg, 515±33 µm, 0.311±0.208 and 22.94±3.09 kg/m2, respectively. There were 2096 (93.6%) grade 0, 94 (4.2%) grade I and 34 (2.2%) grade II basal ganglia lesions. All basal ganglia lesions included lacunar infarctions.
Proportion and CDR class in each decade by gender are shown in online supplemental table 1.
Supplemental material
In stepwise multiple regression analysis, brain infarct lesions including thalamus, cerebellar white matter, cerebellar grey matter, cerebral white matter, cerebral grey matter, paralateral ventricles, mesencephalon and pons, as well as any deeper areas affected by lacunar infarctions other than basal ganglia lesions, were not found to be significant determinants of greater VCDR after adjusting for age groups and gender (basal ganglia lesions: F value=5.01, p=0.0252) (online supplemental table 2).
The proportions of the basal ganglia lesions in each decade by gender are shown in short summary in table 2 and online supplemental table 3.
Age-specific proportion and grades of basal ganglia lesions in this study participants with head MRI (n=2239) (Cochran-Mantel-Haenszel statistic)
The grade of the basal ganglia lesions in both men and women was found to be positively linked to significantly older age groups (male: p<0.0001, female: p<0.0001, respectively; Cochran-Mantel-Haenszel test). In addition, the grade of basal ganglia lesions was found to be significantly higher in men than in women after adjusting for age groups (p<0.0001; analysis of variance). The relationship between VCDR and the ganglia lesion groups was analysed in a general linear mixed model after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, optic disc area, BMI, CCT and history of cataract surgery. Significant relationships were found between VCDR and the degree of basal ganglia lesions, and IOP after adjustment for influential factors (table 3 and online supplemental table 4).
Statistical results of relationship between vertical cup-to-disc ratio and several explanatory valuables in general linear mixed-model analysis after adjusting for age, sex, optic disc area, systolic blood pressure, diastolic blood pressure, body mass index, central corneal thickness and previous cataract surgery
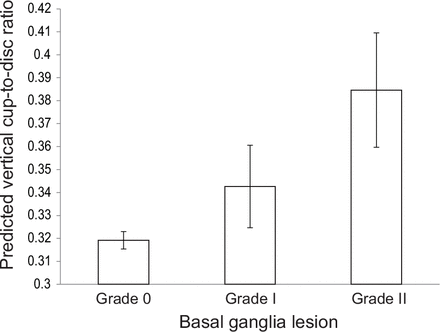
The parameter estimates for VCDR were 0.0071 per 1 mm Hg (IOP). For the basal ganglia lesions, it was −0.0653 for subjects with grade 0 and −0.0419 for subjects with grade I, as compared with the subjects with grade II. These findings indicate that VCDR significantly increased with high IOP and a high degree of the basal ganglia lesions. The predicted VCDR for grade 0, grade I and grade II lesions was 0.319±0.004, 0.343±0.018 and 0.385±0.025, respectively (figure 2). A significant positive linear trend was found between predicted VCDR and the degrees of the basal ganglia lesions (p value trend=0.0096).
The predicted vertical cup-to-disc ratio (VCDR) when the grade of basal ganglia lesion varied from grade 0, I or II, as calculated by linear mixed model. Predicted VCDR (grade 0: 0.319±0.004 (n=2096); grade I: 0.343±0.018 (n=94); grade II: 0.385±0.025 (n=49)) has a significant positive trend with the degrees of the basal ganglia lesions (p value trend=0.0096). The vertical box represents the mean. The thin vertical line indicates the SEM.
Discussion
In this study, we aimed to identify the possible risk factors for elevated optic disc cupping and elucidate the association between several brain lesions and optic disc cupping by analysis of an age/gender-stratified population.
As illustrated in table 2, we observed a higher proportion of basal ganglia lesions in the older age group. Conversely, there was a lower proportion of basal ganglia lesions in the younger age group. Thus, there appeared to be a significant positive relationship between a high grade of basal ganglia lesions and age. In addition, the grade of basal ganglia lesion was higher in men than in women after adjusting for age groups. All of the basal ganglia lesions consisted of lacunar infarctions which occur frequently in the basal ganglia, subcortical white matter and pons.19 Those with less than 200 µm diameter penetrating artery infarcts tended to be asymptomatic.20
Our stepwise multiple regression analysis findings revealed basal ganglia lesions to be the only determinant factor related to elevated VCDR after being adjusted for age and gender. This finding indicates that of the brain infarct lesions, only those in the basal ganglia were significantly associated with elevated VCDR.
In the final multivariate analysis, we observed that VCDR significantly increased with a high degree of basal ganglia lesions and a high IOP after being adjusted for age, gender, optic disc area, systolic blood pressure, diastolic blood pressure, BMI, CCT and a history of cataract surgery. The findings of a previous large epidemiological study showed that the most significant factor for elevated VCDR was high IOP,18 which is also believed to be the most significant factor for glaucoma.13 Thin CCT is reportedly a risk factor for glaucoma, as it has a significant influence on IOP measurement; that is, a thinner cornea has less resistance to corneal shape distortion against external pressure than a thick cornea, and thus leads to the projected IOP reading being less than the real IOP in a thin CCT.21 A previous study has reported that CCT is significantly thinner in normal-tension glaucoma (NTG), defined as a condition in which glaucomatous optic nerve and visual field changes exist within a normal range of IOP, than in POAG or normal subjects.22 In addition, BMI reportedly has a significantly positive correlation with IOP after adjustment for several parameters.23 Despite the correlation with IOP, a high BMI appears to protect against glaucoma, while a low BMI has been found to be a significant risk factor for elevated VCDR and glaucoma.
As for the possible mechanisms, we speculate that BMI and/or CSF pressure mediate the relationship between VCDR and degree of basal ganglia lesions, because BMI alone can probably not fully explain the increased incidence of glaucoma and elevated VCDR. Previous studies have reported a statistically significant correlation between elevated CSF pressure and higher BMI.10 24 A lower CSF pressure caused by low BMI might be a potential risk factor for glaucoma, especially NTG, due to the presence of a pressure gradient across the lamina cribrosa, which is a mesh-like structure in the sclera at the optic nerve head that separates the intraocular space from the CSF-filled subarachnoid space. The translaminar pressure gradient increases with elevated IOP and/or a low CSF pressure.
Recent research suggests a structural and functional relationship between the putamen (a part of the basal ganglia) and BMI. Lou et al reported enlargement in the bilateral putamen in obese and overweight Chinese adults,25 and the authors found that the bilateral putamen volume was positively correlated with both BMI and waist circumference. Obese women and adolescents reportedly show greater responses of the putamen in response to high-calorie food cues compared with their thin counterparts.26 These results provide indirect evidence that elevated VCDR is associated with a low CSF pressure caused by a low BMI, and demonstrate that a low BMI is also associated with a reduced size and lower response of the putamen.
A few neuropathological studies27 28 on human patients with POAG have assessed the brain degenerative changes by using voxel-based morphometry (VBM), a technique that provides the ability to perform automatic and external analysis for structural changes of the whole brain, as well as diffusion tensor imaging. In those studies, VBM and fractional anisotropy revealed a significant volume reduction, not only in optic pathways, including the optic nerve, lateral geniculate nucleus and visual cortex, but also in several brain areas such as the basal ganglia, including the putamen and caudate nucleus. In addition, their findings indicated that neurodegenerative changes beyond that of the optic pathway could be found in patients with POAG, though it remains unclear whether the higher brain cortex is affected or which other regions of the brain are affected in patients with POAG.
It should be noted that the pathophysiology of glaucoma has yet to be fully elucidated. Glaucoma is histologically characterised by RGC apoptosis,29 which is a process of programmed cell death in the absence of inflammation characterised by cell shrinkage, blebbing and DNA fragmentation.30 Major mediators of apoptosis include elevation of glutamate, local oxidative stress caused by elevated IOP, vascular dysregulation and systemic oxidative stress.31 The glutamate transporters excitatory amino acid carrier 1 (EAAC1; encoded by SLC1A1) (known as excitatory amino acid transporters 3 in humans) are highly enriched in the cortex, hippocampus and caudate–putamen, as well as RGCs.32 The RGCs of SLC1A1/EAAC1 knockout mice, known as a model of NTG, reportedly die in response to oxidative stress without elevated IOP and have a high expression of gamma-aminobutyric acid (GABA, a major inhibitory neurotransmitter synthesised from glutamate by glutamic acid decarboxylase in neurons) receptor β subunits.33 In that study, the authors reported that GABAA receptor agonist-induced RGC death is inhibited by bicuculline, a GABAA receptor antagonist. Their findings also showed that hydrogen peroxide-induced death of RGCs is reduced by GABAA receptor antagonist, and that the oxidative stress signalling activated by hydrogen peroxide is also inhibited. The authors concluded that GABAA receptors expressed on RGCs may play a major role in the death of RGCs induced by oxidative stress in glaucoma. Because it is highly expressed in the basal ganglia area of humans as well, the RGCs and basal ganglia may share the same neurodegenerative process from the local and systemic oxidative stress.
It should be noted that this study did have some potential limitations, such as the fact that only subjects from the general Japanese population were enrolled. However, this allowed us to have a higher power of analysis in a single race/ethnicity than in multiple races/ethnicities. On the other hand, considering the different prevalence distribution of glaucoma across races,2 similar considerations may not be applicable to other races/ethnicities. A population-based epidemiological study reported that the Japanese population seems to have a higher incidence rate of NTG.34 It is possible that the subject eyes enrolled in this study have a higher proportion of NTG than eyes in other ethnicities; however, NTG is a common form of POAG, and both share similar mechanisms.35 However, it should be noted that IOP may be a relatively lower risk factor in NTG than in POAG. Considering the proposed mechanism of a translaminar pressure gradient and difficulty of treatment for NTG, we believe there must be other factors that may contribute more in NTG, which led us to the finding of the relationship between VCDR and basal ganglia lesions. Moreover, we do believe that further hospital-based research studies comparing severe glaucoma cases diagnosed with both ocular morphological changes and the development of glaucomatous visual field defects that correspond with the loss of the area of the optic neuronal rim with a healthy control group are surely needed.
In conclusion, the findings in this illustrate that VCDR significantly increases with a high degree of basal ganglia lesions after being adjusted for age, gender, optic disc area, systolic blood pressure, diastolic blood pressure, BMI, CCT and a history of cataract surgery. In this study, we proposed some possible mechanisms to explain this relationship. However, since the detailed mechanism remains unclear, further studies are needed to elucidate how basal ganglia lesions affect optic nerve disc cupping, as well as whether they are a cofactor or causal factor of elevated VCDR. At present, the main treatment of glaucoma is limited to reduction of elevated IOP via the instillation of anti-glaucoma eye drops and/or surgery. It is comparatively difficult to reduce IOP in NTG due to the already normal IOP. These could be important findings that might help shed light on the growing body of evidence for mechanisms and treatments for glaucoma.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The study protocols were approved by the Ethics Committee of the National Center for Geriatrics and Gerontology (899-6), and this study was conducted in accordance with the tenets set forth in the Declaration of Helsinki. Prior informed consent was obtained from all participants.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Conception and design of study—HF, HS and FA. Analysis and interpretation—HF. Writing the article—HF. Critical revision of the article—CT and YN. Final approval of the article—FA, YN, CT, RO, FA and HS. Data collection—RO. Provision of materials, patients or resources—RO. Statistical expertise—CT and YN. Literature search—HF. Administrative, technical or logistic support—HS, Guarantor—HF.
Funding Research funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan (grant number 21-18). This work was also supported in part by the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant number 22H00497).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.