Article Text
Abstract
Objective The Aurolab aqueous drainage implant (AADI) has the potential advantages of less encapsulation and greater cost-effectiveness than the Ahmed glaucoma valve (AGV). The aim of this study was to compare the surgical success and outcomes of the AADI compared to the AGV in Middle-Eastern children.
Methods A comparative retrospective study of consecutive paediatric patients in a tertiary eye hospital was undertaken. Data collected included demographics, type of glaucoma, intraocular pressure (IOP), number of anti-glaucoma medications (AGMs) and any subsequent complications or further surgeries.
Analysis The mean IOP, number of AGMs, surgical success and number of reoperations was compared for the two groups. Surgical success at each visit was defined as IOP of ≥6 mm Hg and ≤21 mm Hg or if the reduction of IOP was ≥20% reduced from baseline.
Results A total of 126 tube surgeries (56 eyes in AADI and 70 eyes in AGV) were performed in patients aged ≤18 years from 2014 to 2019. No difference was observed in the mean IOP between the two groups except at the first month post-operative visit. After six months, the AADI group had a consistently significant lower mean number of AGMs. At last follow-up, 21 (37.5%) eyes in the AADI group were glaucoma medication-free vs 15 (21.4%) eyes in the AGV group (pp=0.047). Kaplan-Meier analysis showed equivalent cumulative probability of success at two years of 69.9% [(45.9%–84.9%)] for AADI vs 66.8% [(53.4%–77.1%])) for the AGV, respectively. Twenty-four eyes in the AGV group needed one or more subsequent surgeries, whereas 13 eyes needed one or more surgery in the AADI group.
Conclusions This study shows an acceptable safety profile for the AADI in children, with a rate of failure that is comparable to the AGV, but less need for glaucoma re-operation or glaucoma medication in the first post-postoperative year.
- glaucoma
- treatment surgery
- intraocular pressure
- child health (paediatrics)
- optic nerve
- aqueous humour
Data availability statement
Data are available upon reasonable request. Data are available at reasonable request by contacting the IRB Department: IRB@kkesh.med.sa
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
The Aurolab aqueous drainage implant (AADI) is a relatively new non-valved glaucoma drainage device (GDD) that has been shown to be safe and effective in the paediatric and adult populations in India, the country of origin of this device.
What are the new findings?
This is the first study in the literature, emanating from outside of India, that provides the safety profile of AADI relative to the Ahmed valve in children.
We found an acceptable safety profile for AADI in children, lower cost compared with other GDDs, with a less need for glaucoma reoperation or glaucoma medication in the short term, compared with the Ahmed glaucoma valve.
How might these results change the focus of research or clinical practice?
AADI is an alternative GDD that can be used in children and has the advantages of cost-effectiveness and safety.
Introduction
Glaucoma drainage devices (GDD) are usually reserved for glaucoma refractory to filtering surgery.1 Currently, the Ahmed glaucoma valve (AGV; New World Medical, Rancho Cucamonga, California, USA) and the Baerveldt glaucoma implant (BGI; Advanced Medical Optics, Santa Ana, California, USA) are the most commonly used GDDs worldwide.2 3 The Aurolab aqueous drainage implant (AADI) is a relatively new non-valved GDD manufactured by Aurolab, a manufacturing division of the Aravind Eye Institute, Madurai, India. The use of this device, which has gained a European conformity (CE) mark, has been shown to be safe and effective in the paediatric and adult population.4 5
The potential advantages of the AADI over the AGV are twofold. First, the AADI is significantly more cost-effective, costing around five times less than the AGV. There is a substantial price difference when comparing different types of GDD available in the market. The AGV costs approximately US$255 and the BGI costs US$750, while the cost of the AADI is about US$50.4 6 Second, non-valved implants in children may offer better long-term glaucoma control compared with valved implants.1 3 7
The AADI has shown a higher success rate and significantly lower intraocular pressure (IOP) and number of antiglaucoma medications (AGM) required postoperatively when compared with the AGV in adults.2 5 6 Similarly, in paediatric glaucoma, the AADI has shown greater complete success with better IOP control and less number of AGMs.3 However, while studies showed similarity or superiority of the AADI compared with the AGV,2 3 5 8 the majority of reports have emanated from India, the country of origin of AADI, and studied Indian eyes only.2–6 8–11 Of the two studies in Middle Eastern populations, one conducted in a mixed adult and paediatric population reported favourable outcomes,12 while another in younger children reported a high adverse event profile.13
The aim of the current study was to compare the efficacy and safety of the AADI with the AGV in a Middle Eastern paediatric population with refractory glaucoma.
Methods
Study design
This was a retrospective cohort study undertaken from December 2019 to August 2020 at King Khaled Eye Specialist Hospital (KKESH), a tertiary eye care institute in Riyadh, Saudi Arabia. All children with refractory glaucoma who received the AADI in KKESH in one or both eyes from July 2014 until November 2019 and were aged ≤18 years at the time of implant were included. Consecutive children who received the AGV implant during the same period were taken as a comparison group. There were only a few children who had bilateral implants. In such cases, both eyes were included in order to maximise the sample size, but a mixed methods statistical approach was employed to account for intereye correlations (see the Data analysis section), as well as adjust for differences in baseline characteristics.
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Inclusion and exclusion criteria
All patients aged 18 years or under at the time of GDD surgery were included. We excluded children who underwent combined procedures (eg, cataract, combined with GDD surgery). Children were not excluded based on diagnosis subtype or lens status.
Data collection
We identified children who received the AADI or AGV implant using operative codes and the logbook of surgical implants. Baseline information collected included basic demographic information such as age and gender of the child, systemic diagnoses, type of glaucoma, previous surgery, visual acuity, lens status, central corneal thickness and preoperative IOP, and number of AGMs. The subtype of glaucoma was categorised according to the classification published after the ninth meeting of the World Glaucoma Association Consensus in the childhood glaucoma.14 The operative details included the type of implant used, use of a ripcord and ligature, location of tube insertion (anterior chamber, sulcus, pars plana) and the type of patch used (sclera, pericardium or cornea).
Postoperatively, we documented the IOP at each visit, the presence and type of complications, the number of AGMs, and the type and timing of subsequent surgeries.
Outcome measures
Surgical success at each visit was defined as IOP of ≥6 mm Hg and ≤21 mm Hg or if the reduction of IOP was ≥20% from baseline.15 Failure was defined if any of the following has occurred: (1) IOP was more than 21 on two consecutive follow-up visits; (2) use of systemic AGM (oral acetazolamide) to control IOP; (3) loss of perception of light; (4) need for a further glaucoma surgery to control IOP; and (5) persistent hypotony, defined as IOP less than 6 mm Hg on two consecutive visits more than 1 week apart. Failure by the IOP criterion was only considered to occur a minimum of 3 months postsurgery, in accordance with large randomised trials of non-valved glaucoma implant surgery.15 16 Further glaucoma surgery to control the IOP included a new glaucoma procedure (eg, another tube surgery or cyclodestructive procedure) or reoperation of the same GDD to control IOP (eg, removal of encapsulation). Ripcord removal, tube repositioning, tube trimming and other minor interventions—even if performed in the operating theatre—were not considered as a failure per se but were elaborated in the analysis of subsequent surgeries. A hypertensive phase was defined as IOP greater than 21 mm Hg during the first 3 months that was not attributed to identifiable causes of high IOP (such as blockage by vitreous or blood) and patients who had ligated tube and had IOP more than 21 mm Hg during the early postoperative phase.
Surgical technique and postoperative care
AGV and AADI were performed by four different surgeons with the same technique as follows: after sterile draping, speculum and a 7-0 vicryl (Coated Polyglactin 910 Violet; Ethicon, Johnson and Johnson, USA) corneal traction suture to infraduct the eye, a fornix-based conjunctival flap was dissected in the planned quadrant and eraser cautery (disposable, 18-gauge, non-stick bipolar pencil; Kirwan Surgical Products, Marshfield, USA) performed. Blunt dissection was used to open the exposed quadrant. The implant (AADI/AGV) was primed with a balanced salt solution and the plate inserted into the exposed quadrant. For the AADI, a 5-0 or 4-0 nylon (Monofilament Polyamide 6, Black; Ethicon, Johnson and Johnson) suture was placed in the lumen of the tube as a ripcord and two 7-0 vicryl sutures were used to occlude the tube in watertight fashion, with an additional 9-0 nylon (Ethicon) in cases considered high-risk for hypotony. The ripcord suture was placed in the subconjunctival space usually in the inferotemporal quadrant at the end of the surgery (for later retrieval usually at 3–6 months after surgery).
The plate was sutured to the sclera with 9-0 prolene (Monofilament Polypropylene Blue; Ethicon, Johnson and Johnson) or 9-0 nylon (Ethicon) with the anterior edge 8–9 mm posterior to the limbus for the AGV and 9.0–10.0 mm posterior to the limbus for the AADI. A paracentesis was performed and the anterior chamber filled with a small amount of viscoelastic for AGV, but viscoelastic was avoided for AADI to avoid postoperative IOP rise. In case of AADI ligation (7-0 vicryl) and ripcord (4-0 nylon) were applied in a watertight fashion. A 30-gauge guiding followed by a 23-gauge needle was used to make a stab incision approximately 2 mm posterior to the limbus for the tube to be positioned near the iris. The tube was trimmed to an appropriate length and then inserted into the anterior chamber. The tube was checked to be in a good position, close to the iris, and length adjustment was made to avoid proximity to the corneal endothelium. The tube was sutured to the episclera with 9-0 prolene or 9-0 nylon. Tube fenestrations were made for the AADI, usually two to three, with a 7-0 vicryl needle. A piece of donor pericardium (Tutoplast; Tutogen Medical, Germany), sclera or cornea was trimmed and sutured to the episclera to cover the tube and entry site. The conjunctiva was closed with 9-0 vicryl continuous sutures and additional sutures were applied when needed to have a watertight conjunctival closure at the end of the surgery. The subconjunctival space was injected with 0.5 mL of cefazolin (100 mg/mL; Zolecin, HIKMA Pharmaceuticals, Amman, Jordan) and 0.5 mL of dexamethasone sodium phosphate (4 mg/mL; Egyptian Int Pharmaceutical, Egypt).
All children were treated with moxifloxacin 0.5% eye-drops (Vigamox Ophthalmic Solution; Alcon, Fort Worth, Texas, USA) for 2 weeks and prednisolone acetate 1% drops (Pred Forte; Allergan, New Jersey, USA) tapered over 4–6 weeks, and atropine sulfate 1% drops (Bausch & Lomb, Aubenas, France) were used two times per day for 2–3 weeks. AGMs were resumed with time at the discretion of the clinician, depending on the IOP and level of optic nerve damage.
All children were admitted for surgery and then discharged after 1–3 days after the surgery, after which children were followed at 1–2 weeks, then 1 month, then every 3 months for the first year. The exact timing of visits depended on the postoperative course, IOP and presence of any complications.
Data analysis
No patient was excluded based on follow-up time, and all data, until the last available follow-up, were used for analysis. IOP, medications and survival analysis were computed at each time point with all the available data.
Data were entered using Microsoft Excel V.2010 (Microsoft Corporation, Redmond, Washington) and analysed using STATA V.16.1. Means with SD were calculated to describe continuous variables, whereas counts and percentages were used to describe categorical variables. χ2 test or Fisher’s exact test was used to compare proportions, as appropriate.
Postoperative IOP and AGM data were categorised into day 1 (acceptable time 1–3 days), week 1 (4–14 days), month 1 (15–60 days), month 3 (61–122 days), month 6 (123–272 days), month 12 (273–456 days), month 18 (457–639 days) and month 24 (640–913 days).17
Kaplan-Meier curves were plotted to compare the cumulative probability of failure in the two groups (AADI/AGV). The log-rank test was used to calculate the p values. Cox proportional hazards regression analysis was used to identify factors associated with treatment failure.
Further, mixed effect models were developed to assess the effect of AADI versus AGV on IOP and AGM use over 2 years while adjusting for age, gender, glaucoma type, prior tube surgery and lens status. The base model (model 1; online supplemental table 2) included IOP as a dependent variable and treatment and its interaction terms with time as explanatory variables. Next, the model was adjusted for age, gender, glaucoma type, prior tube surgery and lens status. A similar process was followed to develop models with AGM as a dependent variable (online supplemental table 3). STATA’s margins command was used to compute adjusted predicted IOP and AGM values for both the AADI and AGV groups. From the fitted models, we graphed the change in IOP and AGM at 3, 6, 9, 12, 15, 18 and 24 months after the surgery.
Supplemental material
A p value <0.05 was considered statistically significant.
Results
A total of 126 tube surgeries were performed for 119 patients. The eligible sample consisted of 56 eyes in the AADI group and 70 eyes in the AGV group. Both groups had similar baseline characteristics, except for the lens status. A significantly higher proportion (p=0.016) of eyes in the AGV group were aphakic (41.4%) compared with the AADI group (19.6%) (table 1). The mean duration of follow-up was significantly (p=0.001) longer in the AGV group (25.33±11.03; range: 4.74–55.07 months) compared with the AADI group (13.77±10.07; range: 0.82–40.10 months). Further details of the demographic and baseline characteristics of both groups are illustrated in table 1. Among all study subjects, only four eyes (7.1%) had no previous ocular surgery (online supplemental table 1).
Demographic profile and baseline characteristics of patients in the two treatment groups
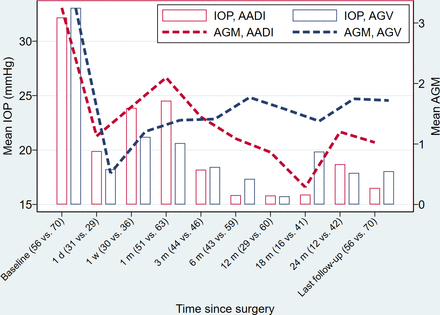
Figure 1 shows the IOP across all time points for the two implants. Both groups had a comparable decrease in mean IOP at all postoperative visits, with a mean reduction of 45.0% in the AADI group and 46.5% in the AGV group at the first year postoperative visit. This percentage of mean reduction was maintained to a similar level at the last visit (45.7% and 44.7% for AADI and AGV, respectively).
Mean IOP and mean AGM trend over the study period. AADI, Aurolab aqueous drainage implant; AGM, antiglaucoma medication; AGV, Ahmed glaucoma valve; IOP, intraocular pressure.
No significant difference was observed in the mean IOP between the two groups at any particular visit except at the first month postoperative visit, where the mean IOP was higher in the AADI group (24.1±10.2 mm Hg) compared with the AGV group (20.7±6.7 mm Hg) (p=0.043), due to the tube ligation during this period.
The mean number of AGM was higher in the AADI group, compared with the AGV group, in the initial postoperative period. However, at 3 months, both groups had a similar mean number of AGM: 1.6 (95% CI 1.4 to 1.9) for AADI vs 1.6 (95% CI 1.6 to 2.0) for AGV. After 6 months, the AADI group had a consistently significant lower mean number of AGMs (online supplemental figure 1). At the last follow-up, 21 (37.5%) eyes in the AADI group were glaucoma medication-free vs 15 (21.4%) eyes in the AGV group (p=0.047). Findings of the linear mixed effects analysis of the effect treatment (implant) on IOP and on AGM over time while adjusting for other factors are shown in online supplemental tables 1 and 2, figure 1.
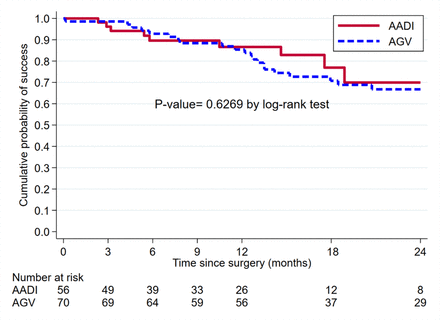
Kaplan-Meier survival analysis (figure 2) showed that the AADI and AGV groups had an almost identical cumulative probability of success at 1 year, 86.6% (95% CI 72.2% to 93.8%) for the AADI and 85.4% (95% CI 74.5% to 91.9%) for the AGV, as well as at 2 years: 69.9% (95% CI 45.9% to 84.9%) vs 66.8% (95% CI 53.4% to 77.1%), respectively.
Kaplan-Meier survival curve showing cumulative survival for Ahmed glaucoma valve (AGV) and Aurolab aqueous drainage implant (AADI) in children.
A Cox proportional hazards regression analysis did not reveal any specific factors associated with treatment failure. In particular, the type of GDD (AADI or AGV) was not associated with failure. The primary reasons for failure were inadequate IOP, reoperation for glaucoma and No light perception (NLP) vision, in the following frequencies: 5 (55.6%), 3 (33.3%) and 1 (11.1%), respectively, in the AADI group, and 12 (46.2%), 14 (53.8%) and 0, respectively, for AGV failures. None of the eyes had persistent hypotony in the study period, and subsequently none of them failed due to hypotony.
The hypertensive phase was more common in the AADI group, 27 eyes (48.2%) vs only 15 eyes (21.4%) in the AGV group. Table 2 illustrates the number and rates of all postoperative complications. Overall, the number of complications was similar in both groups. However, there were some differences: the AADI group had a significantly (p=0.022) higher rate of transient choroidal detachment (12.5%), while AGV had significantly (p=0.033) higher incidence of late encapsulation. Across groups, choroidal detachment occurred in eight eyes (four pseudophakic eyes, three aphakic eyes and one phakic eye).
Complications over the entire follow-up*
Over the study period, 34 (60.7%) eyes in the AADI group underwent subsequent surgeries, consisting of ripcord removal (73.5%), tube trimming (20.6%) and other surgeries (8.8%). This latter group included removal of a previous AGV due to exposure, phototherapeutic keratectomy, and pupilloplasty with transpupillary membranectomy (one surgery each). On the other hand, only 26 (37.1%) eyes had subsequent surgeries in the AGV group; among them, 10 eyes (38.5%) had another glaucoma surgery (AADI in 3 eyes, cyclophotocoagulation, CPC, in 6 eyes, endocyclophotocoagulation, ECP, in 1 eye) and 6 eyes (30.8%) had other ocular surgeries (including 1 therapeutic penetrating keratoplasty for microbial keratitis, 1 amniotic membrane transplantation, then a retrocorneal membrane excision, then a Boston keratoprosthesis, 1 corneal resuturing post open globe, 1 corneal tattoo, 1 secondary intraocular lens (IOL), and 1 optical iridectomy; ie, a total of 8 surgeries). Table 3 summarises all subsequent surgeries.
Subsequent surgeries over the entire follow-up*
Discussion
The aim of the current study was to provide an independent report regarding the surgical outcomes of the AADI relative to the AGV in Middle Eastern children with refractory glaucoma. This aim was relevant to our practice given that the AGV is the most commonly used GDD in the Middle East and the AADI is the contemporary and more cost-effective alternative.
Although the mean IOP was almost similar postoperatively for the two implants, children who received the AADI had a lower number of AGMs and a lower number of de novo subsequent glaucoma surgeries after the GDD. Similar outcomes have been observed in other studies that showed comparable results or superiority of the AADI when compared with the AGV in both adults and children.2 3 5 6 8 18 In children, Kaushik et al4 found that the AADI had a cumulative probability of success of 91.2% at 6 months and 81.7% at 18–24 months. Senthil et al3 reported for the AADI group a cumulative probability of qualified success of 91.6% at 12 months and 81% at 36 months, while for the AGV group 88.1% at 12 months and 85% at 36 months, with no statistical significance, but complete success was significantly higher in AADI. Similar findings were observed in large controlled trials in adults that compared non-valved BGI with AGV. They found higher success, less de novo subsequent glaucoma procedures and fewer glaucoma medications with lower IOP in non-valved BGI.19 Fewer AGMs can be attributed to less encapsulation of the AADI in children compared with the AGV. The AGV can fail in children due to encapsulation or growth of fibrous membranes within the valve.20
Being a retrospective study, there were differences in some baseline characteristics between the AGV and AADI groups. There were 20 of 56 eyes in the AADI group that already had a prior GDD compared with 7 of 70 eyes in the AGV group. While on one hand the two groups may be difficult to compare, it also highlights that a significant proportion of children who had a previous AGV are likely to need a non-valved implant for IOP control. Further, second tubes would be expected to function less well compared with the first tube, given the tendency of these children for encapsulation and scarring, but our study still pointed to equivalent or better IOP control in the AADI group, thus adding to the finding that AADI may achieve better glaucoma control in the paediatric population.
Regarding device safety, Rateb et al13 raised concerns over the use of AADI in children after observing an intense inflammation developed after using AADI in children, and this was attributed to the material that AADI is made of. Although both AGV and AADI are made of silicone, AADI’s manufacturer call it ‘permanent implantable grade silicone’,21 while AGV and BGI call it ‘medical grade silicone’.22 23 Silicone material is a broad term, and implantable medical devices can be classified as medical non-implantable and short-term and long-term implantable.24 To avoid ambiguity of the degree of biocompatibility, there should be a clear disclosure regarding the type and grade of silicone. In addition, a unified terminology on the grades of silicone must be used. A well-known standard is the US Pharmacopoeia classification of plastics, which has six grades to label a material according to structured and specified biocompatibility challenge tests.25 Using such standards will limit concerns about the biocompatibility of the materials that are used in such implants.
We found only one child who developed trans-pupillary membrane over a hydrophilic acrylic intraocular lens (HAIOL) 18 months after AADI surgery and all the other 55 children did not show marked inflammation or specific types of complications. There was no obvious reason why this specific patient developed this late membrane formation (such as uveitis or poor compliance to medications). In addition, in all the postoperative visits there was no evidence of unexpected or intense inflammation. Ahn et al26 reported a case of severe prelenticular membrane formation over a HAIOL in early postoperative days after cataract surgery in an eye with an AGV and assumed HAIOL might interact with silicone valve. Our observations in this study suggest that AADI is a safe device to be used in paediatric patients and that this is supported by other studies that used it in adults and paediatric age groups.2–6 8 10 13 18 27–31 In fact, the frequency of adverse events for the AADI was similar to the AGV in our study (table 2), apart from a higher incidence of transient early postoperative choroidal detachment.
Despite the less need for AGV with the ADDI compared to the AGV, there are several inherent advantages of using a valved GDD. First, as demonstrated in this study and others, the incidence of postoperative hypotony is less.3 8 16 19 32 In our study, seven children developed transient choroidal detachment in the AADI group compared with only one in the AGV group. All the eight patients had resolution of the choroidal detachment without any surgical intervention and none of them persisted more than 2 months. In addition, no serious complication was observed in these patients, such as cataract or persistent decrease of vision. Second, AGV does not, like the AADI, require removal of the ripcord, which in younger children necessitates a second general anaesthetic. This should be balanced against the risk of needing further glaucoma surgery: in this study 25 children required a further glaucoma surgery in the AGV group compared with only 2 in the AADI group.
A variety of factors may influence the choice of GDD in a given centre, in addition to efficacy and safety and compliance to follow-up, such as the availability of the implant, the surgeon preference and the cost/affordability of the device. One study mentioned ‘AGV costs approximately US$255 and BGI costs US$750, while the cost of AADI is about US$50’.4 6 In Saudi Arabia, the cost is around US$520 for both BGI and AGV, and AADI costs $150. This cost difference with comparable outcomes or even the superiority of AADI is another incentive for continued use in children.
There are several limitations to this study. First, the follow-up in the AADI group was significantly less than the AGV group. Follow-up for the AADI is limited by the fact that we started implantation of this device in 2016, whereas AGVs had been implanted for many years before then. Since 2017, there has been a complete shift from BGI to AADI in 2017 in our institution due to the safety and cost-effectiveness of the AADI that we noted. We did not exclude any child on the basis of follow-up, since excluding patients with shorter follow-up (less than 6 months) might bias the outcome. For instance, we noticed that few patients in our study failed within the first 3 months and had subsequent glaucoma surgery, so these patients would have been automatically excluded if we had specified a minimum of 6 months of follow-up. A second limitation of a retrospective study, such as this, is selection bias. In other words, the baseline factors are often dissimilar between the two groups. In this regard, we noted that the proportion of children in the AGV group who were aphakic at the time of GDD was higher than the AADI group. Aphakia is considered a risk factor for hypotony,33–35 and this was the reason for a higher number of aphakic children in the AGV group, as surgeon preference is to use a valved implant over non-valved AADI because non-valved implants have higher hypotony and hypotony-related complications.19 However, in order to adjust for differences in baseline characteristics and follow-up time, we also employed a multilevel model to predict relative efficacies, in terms of IOP and AGM use for the two implants after adjusting for differences. This analysis showed that the two implants have comparable IOP-lowering over the first 2 years, with children receiving the AADI requiring less AGMs. Another limitation is that the surgeries were performed by different surgeons. However, the technique is standardised in our department, with all surgeons using a ripcord and vicryl ligature for the AADI to minimise the risk of hypotony.
Despite limitations, we are probably one of the few centres globally outside of India able to provide data on the efficacy and safety of this implant in a paediatric population, as the AADI is not Food and Drug Administration-approved for use in the USA. In the Middle East both AGV and non-valved implants are being used. Our study focused on the outcomes of a procedure for a condition that is uncommon in other settings and our analysis controlled for several potentially confounding variables. Although our study has larger numbers compared with previous studies of the AADI versus the AGV,2 3 6 a minimum of 356 eyes per group would be powered (80%, alpha 0.05) to detect a 10% difference in survival function between the two groups. Therefore, the results of our study should also be interpreted with some caution.
Long-term outcomes are valuable in any glaucoma study, as failure rate inevitably increases with the number of years postsurgery. Further studies, with larger sample size and longer follow-up, are needed to confirm our findings and to study relative longer-term failure rates.
In conclusion, this study shows an acceptable safety profile for the AADI in children, with a rate of failure that is comparable with the AGV, but less need for glaucoma reoperation or glaucoma medication in the early postoperative period of 1 year. Adequately powered studies are needed to verify our findings.
Data availability statement
Data are available upon reasonable request. Data are available at reasonable request by contacting the IRB Department: IRB@kkesh.med.sa
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Institutional Review Board of King Khaled Eye Specialist Hospital (IRB no 1950-R) and adhered to the principles of the Declaration of Helsinki.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors have made substantial intellectual contribution to the study design, conceptualisation of the study, writing the initial paper or approving the final version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.