Article Text
Abstract
Objective We examined the production of prostaglandin E2 (PGE2), which is the key prostaglandin involved in inflammatory disorders of the ocular surface. Tears and conjunctival fibroblasts were evaluated in order to assess allergic inflammation and the effect of specific drugs.
Methods and analysis PGE2 was measured in tears from both patients and normal volunteers. Primary cultures of human conjunctival fibroblasts were incubated with interleukin (IL)-4 and tumour necrosis factor (TNF)-α with or without ketotifen fumarate or dexamethasone. The culture supernatants were removed 24 hours after exposure and the concentrations of PGE2 were quantified by ELISA.
Results Significantly higher levels of PGE2 were observed in the tears of patients with severe allergic conjunctivitis than in those with post-surgical inflammation (p=0.02), and this production was reduced by eye drops. Stimulation with IL-4 and TNF-α induced the generation of PGE2 in supernatants of conjunctival fibroblasts, and this production was significantly downregulated by ketotifen fumarate or steroids.
Conclusion PGE2 may participate in the pathogenesis of severe ocular allergic disease, and both ketotifen fumarate and steroid reduce the production of PGE2.
- tears
- treatment medical
- inflammation
- drugs
- conjunctiva
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Prostaglandin E2 (PGE2) is produced in the tears of patients with inflammatory disorders in the ocular surface as severe allergic conjunctivitis.
What are the new findings?
Significantly higher levels of PGE2 were observed in the tears of patients with severe allergic conjunctivitis. Moreover, ketotifen fumarate affected PGE2 production and reduced allergic inflammation.
How might these results change the focus of research or clinical practice?
Prostaglandins have potential importance in the management of ocular allergic inflammation and could be the pathogenesis of severe ocular allergic disease.
Introduction
Evidence suggests that ocular surface tissues such as the cornea and conjunctiva play active roles in ocular allergy; histamine receptors are expressed on conjunctival epithelial cells, and proinflammatory cytokines such as interleukin (IL)-4 and tumour necrosis factor (TNF)-α are released in tears in ocular allergic inflammation.1 2 Arachidonic acid (AA), released from cell membrane phospholipids of activated cells, is metabolised to prostaglandins (PGs) by the sequential activity of cyclooxygenase (COX).3 It is known that corneal and conjunctival cells also release AA in inflammatory conditions.4 We have previously reported that PGE2 is produced by human conjunctival and corneal cells in response to IL-4 and TNF-α stimulation.5 In this report, we analysed PGE2 production, which has been thought to contribute to the development of inflammation, in allergic inflammation within two representative types of ocular tissues: epithelial cells and fibroblasts.5 PGs have been reported to mediate various inflammatory responses in ophthalmological diseases.6 COX-2 is an inducible enzyme and highly associated with ocular surface injections.7 8 TNF-α is a potent inducible inflammatory cytokine and is capable of inducing PG production by cells.9–12 Corneal keratocytes produce chemokines such as regulated on activation normal T cell expressed and secreted (RANTES),13 monocyte chemotactic protein (MCP)-1,14 15 and eotaxin16 following stimulation by IL-4 and TNF-α. These chemokines recruit eosinophils into local tissues. In our previous study, stimulation with IL-4 and TNF-α also affected the expression of many other genes in keratocytes, including those of various chemokines, such as IP-10,17 the fourth most induced transcript I-TAC,17 the sixth most induced transcript RANTES18 and the ninth most induced transcript MCP-2. Activated conjunctival epithelial cells release biologically active compounds which play essential roles in the pathogenesis of ocular inflammation, including lipid mediators, eicosanoids, and a variety of cytokines and chemokines.
Ketotifen fumarate is non-competitive antagonist of the histamine H1 receptor and a mast cell stabiliser, which inhibits the release of inflammatory mediators from mast cells. In addition, its ophthalmic solution was safe, well tolerated, and effective in preventing the signs and symptoms of allergic conjunctivitis.19
Although previous ocular allergy studies have provided useful insights, the specific mechanisms of the regulated drug processes in severe ocular allergic diseases are not fully understood. In this study, we examined PGE2 production in the tears of patients with severe ocular allergic inflammation, followed by primary cultures of the human conjunctival fibroblasts.20 This study aimed to investigate the effects of ketotifen fumarate and steroids on the release of PGE2 by primary cultures of human conjunctival fibroblasts.
Materials and methods
Written informed consent was obtained from all subjects before participation. Patient data were anonymised before access and analysis.
Clinical samples
Five patients who underwent simple penetrating keratoplasty (PKP) surgery (mean age: 27.7±9.1 years) were recruited to obtain samples 1 hour post-surgical inflammation. Four patients had been performed PKP due to keratoconus, and the other had been due to bullous keratopathy. We chose PKP case as disease control because under the epithelial defect, continuous stimulation of stromal cell may increase the production of PGE2.5 In addition, five patients (mean age: 15.1±3.4 years) with atopic keratoconjunctivitis or vernal keratoconjunctivitis (VKC) with corneal erosion were recruited to obtain samples exhibiting severe allergic inflammation.21 Their treatment included Zaditen (0.05% ketotifen fumarate, Santen, Osaka, Japan) and Santeson ophthalmic solution (0.1% dexamethasone, Santen, Osaka, Japan), both administered four times per day for 4 weeks. We included an additional five participants as normal controls (mean age: 31.6±4.3 years). The diagnosis of atopic dermatitis was made by a dermatology specialist based on the criteria of Hanifin et al.22 No treatment was supplied prior to tear sample collections. In addition, samples of normal cases were collected from normal volunteers. Samples were collected before and 4 weeks after topical treatment in the control group, and a day after the operation in patients with PKP. Using a micropipette, 20 µL of tears were collected at the lateral canthus of the eyelid without anaesthetic; patients were in the supine position with their heads tilted to the side. Tear samples were centrifuged immediately at 4°C to remove cells and transferred to new tubes. Tear samples were stored at −80°C until further examination.
Measurements
PGE2 concentration was measured by ELISA (R&D, Minneapolis, Minnesota, USA). The cytokines, RANTES and IL-8 were purchased from R&D. Eotaxin was received as a gift from Dr Hiroshi Kawasaki of the Department of Clinical Immunology, The Institute of Medical Science, University of Tokyo, Tokyo, Japan.
Primary culture
Human conjunctival samples were collected using scissors from normal volunteers from whom informed consent had been obtained. Samples were incubated in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 medium (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to grow fibroblasts. Specimens were cultured in complete medium at 37°C in a humidified atmosphere supplemented with 5% CO2 in air for a few weeks. The cells were grown with incubation in a flask. Fibroblasts were subcultured in 96 well plates at a concentration of 1×105 cells/mL. One day later, cells were incubated with TNF-α (30 ng/mL) and IL-4 (30 ng/mL) in combination with ketotifen fumarate and dexamethasone: 10−6~−8 M (Sigma-Aldrich, St. Louis, Missouri, USA). Twenty-four hours later, culture supernatants were collected and PGE2 levels were determined.
Statistical analysis
The data were analysed using Prism software (V.6.04 for Mac; GraphPad Software, San Diego, California, USA). The D’Agostino-Pearson omnibus normality test was used to assess whether the data showed a normal distribution. Student’s t-test was performed to assess the differences in tear and PGE2 production associated with IL-4 and TNF-α. The same test was also used to assess differences in drug inhibition associated with ketotifen fumarate and dexamethasone. Values are expressed as mean±SD. P values were not adjusted for multiple comparison testing. Values below 0.05 were considered statistically significant.
Results
Mean tear PGE2 levels are shown in figure 1; the level was 13.0±3.6 pg/mL in post-PKP inflammation, 6.0±4.2 pg/mL in normal controls and 190±100 pg/mL in the severe allergic inflammatory condition, which decreased significantly to 62.6±43.3 pg/mL after 4 weeks of treatment with ketotifen fumarate and dexamethasone (p=0.04).
PGE2 production in the tears of patients, and its downregulation by ketotifen fumarate and dexamethasone. The mean production of PGE2 in the tears of patients with severe allergic inflammation (n=5) was significantly higher than that in patients following 4 weeks of eye drop treatment, in patients post-surgical inflammation (n=5) and normal controls (n=5). Results are presented as means±SD. Student’s t-test was performed. Allergy indicates patients with severe allergic inflammation. PKP indicates patients with post-surgical inflammation. PGE2, prostaglandin E2; PKP, penetrating keratoplasty.
Patients with severe allergic inflammation produced significantly higher levels of PGE2 than those found after 4 weeks of topical treatment (0.05% ketotifen fumarate and 0.1% dexamethasone), and in those post-PKP inflammation, and in normal controls (p<0.05; figure 1).
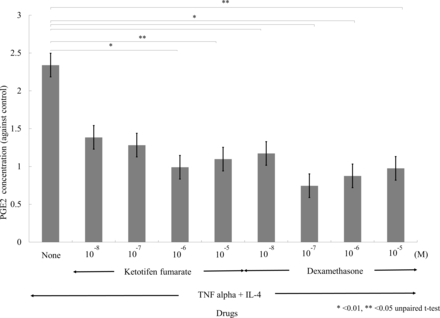
Stimulation with 30 ng of IL-4 and TNF-α for 24 hours induced the production of PGE2 by fibroblasts, and this production was significantly downregulated by ketotifen fumarate at concentrations of 10−6 M (p<0.01) and 10−5 M (p<0.05). Dexamethasone reduced the PGE2 by fibroblasts at concentrations of 10−8 M, 10−7 M and 10−6 M (all p<0.01), as well as 10−5 M (p<0.05; figure 2). Chemokines such as IL-8, RANTES and eotaxin did not induce the production of PGE2 by fibroblasts (figure 3).
Effects of ketotifen fumarate and dexamethasone on PGE2 production by conjunctival fibroblasts. Human conjunctival fibroblasts were maintained in 96 well plates at a concentration of 1×105 cells/mL. Cells were incubated with IL-4 (10 ng/mL or 100 ng/mL) and/or TNF-α (10 or 100 ng/mL) and harvested 24 hours later. Ketotifen fumarate and dexamethasone were added at concentrations from 10−8 nM to 10−5 nM. The ‘None’ bar indicates medium alone. The generation of PGE2 was determined in cell-free supernatants by ELISA. Results are presented as means±SD of seven independent experiments. Significant differences at the levels of p<0.05 and p<0.01 vs time 0 are indicated by * and **, respectively (Student’s t-test). IL-4, interleukin 4; PGE2, prostaglandin E2; TNF, tumour necrosis factor.
PGE2 production by conjunctival fibroblasts stimulated with chemokines. Human conjunctival fibroblasts were maintained in 96 well plates at a concentration of 1×105 cells/mL. Cells were incubated with IL-8 (30 ng/mL), RANTES (30 ng/mL) and eotaxin (30 ng/mL), and harvested 24 hours later. The left bar ‘0’ indicates medium alone. The generation of PGE2 was determined in cell-free supernatants by ELISA. Results are presented as means±SD of at least three independent experiments. No significant differences were observed between the chemokine combinations (Student’s t-test). IL-8, interleukin 8; PGE2, prostaglandin E2; RANTES, regulated on activation normal T cell expressed and secreted.
Discussion
This study identified the production of PGE2 in tears of patients with severe allergic conjunctivitis, and that this production was decreased by the administration of 0.05% ketotifen fumarate and 0.1% dexamethasone. PGE2 was also produced by primary cultured conjunctival fibroblasts from normal volunteers following stimulation with IL-4 and TNF-α, and this production was also decreased by ketotifen fumarate or dexamethasone. PGs induce vasodilation, which is an important feature of local inflammation.23 AA, which is released from the cell membrane phospholipids of activated cells, is metabolised to PGs by the sequential activity of COX-1 and COX-2.24
It has been reported previously that a conjunctival allergic reaction can be induced with antigen in sensitised guinea pigs, and that in the acute phase of allergic conjunctivitis, the PGE2 levels can be determined in lavage fluid; PGE2 was produced simultaneously in the conjunctiva and exhibited identical profiles of synthesis in response to antigen provocation.25 In this study, PGE2 was identified in the tears of patients with severe allergic conjunctivitis, who were thought to be in the late phase of this condition. Levels of PGE2 were higher in the tears of patients with severe allergy than in tears 4 weeks after eye drop treatment, and in post-surgical patients with PKP. The PKP tear samples, used as inflammatory controls, likely involve quite different inflammatory mechanisms and are not chronic. It is therefore not surprising that PGE2 levels in patients with PKP were close to baseline. While our PKP surgical procedure usually concludes with a subconjunctival steroid injection and coverage with a medical use contact lens, PGE2 production was significantly higher in the tears from patients with a severe allergy. This may due to the corneal ulcers in patients with severe allergic disease.5 PGE2 was produced from the conjunctival fibroblasts by the action of IL-4 or TNF-α in tears,5 as discussed below.
In vitro concentrations of procollagens and inflammatory cytokines in fibroblasts from patients with VKC, including TNF-α and other cytokines, have been shown to be increased.26 TNF-α levels have been shown to be increased in the tears of patients with atopy after conjunctival allergen challenge.11 We have also reported increased levels of IL-4 in tears from patients with VKC.27 We have stimulated ocular surface cells by IL-4 and TNF-α through allergic inflammation in vitro. Cooperation between proinflammatory cytokines is well documented in the literature. Production of PGE2 was induced in primary cultured corneal keratocytes, conjunctival epithelial cells, and fibroblasts by IL-4 and TNF-α. Kinetic studies have demonstrated that PGE2 is significantly increased in a time-dependent manner.5 In this paper, corneal epithelial cells produced almost no PGE2. The reason for this is still unknown, but we hypothesise that corneal epithelial cells did not release PGE2 and may be the target cell of this inflammatory response. Thus, we are now investigating how PGE2 affects corneal epithelial cells. Repeated induction of PGE2 production by conjunctival cells may cause corneal epithelial injection, and once the epithelial barrier is broken, keratocytes could produce more PGE2 and epithelial erosions or ulcers can occur in severe allergic conjunctivitis. The effects of PGE2 on corneal epithelial cells are still unclear. We are studying these effects because we believe the corneal epithelium is a target of PGE2. Any event which induces the production of PGE2 may play an important role in allergic inflammation and result in severe clinical manifestations.
The effect of steroids on COX enzyme inhibition is now widely accepted. The anti-inflammatory effects of ketotifen fumarate are therefore likely to be due to the inhibition of COX. The identification of selective inhibitors of COX-2 will therefore lead to advances in therapy.24 In this study, the production of PGE2 by conjunctival fibroblasts was decreased by ketotifen fumarate, which is an antihistamine drug with an incompletely understood efficacy. Ketotifen fumarate has a strong inhibitory effect of platelet-activating factor (PAF), which enhances AA metabolism in addition to its role in both the acute and delayed phases of allergy. There is one speculation that under the circumstances an environment without mast cells, PGE2 production was suppressed by its PAF inhibitory effect. Although this agent has distinct antihistamine molecular mechanisms of action and exhibits different immunoregulatory profiles, the direct effects on antigen presentation processes remain unknown. This is why we have studied the effects of this drug on primary cultured ocular surface cells. The pathogenesis of topical therapeutic antihistamine drugs in the inhibition of allergic inflammation may be due to other reasons.
CC chemokines, such as eotaxin and RANTES, are important in recruiting eosinophils into tissues affected by allergy.28 RANTES has been found in the tears of patients with allergic conjunctivitis,29 and is produced by conjunctival and corneal cells. We have previously reported that eotaxin is present in the tears of patients with allergy and severe corneal injury, collating with the number of eosinophils in the tears.30 We have also found that IL-4 induces eotaxin production by human corneal keratocytes.16 In this study, we investigated the induction of PGE2 production by fibroblasts associated with IL-8, RANTES and eotaxin. No effects on fibroblasts were observed (figure 3). Chemokines have no direct effects on keratocytes during the induction of PGE2 production. This result implies that PGE2 production by fibroblasts is not promoted by chemokines via autocrine or paracrine stimulations, but instead by the direct stimulation of IL-4 and TNF-α.
In summary, we found that PGE2 was produced in the tears of patients with severe allergic conjunctivitis, and produced by cultured conjunctival fibroblast cells in a sequential manner following stimulation with IL-4 and TNF-α. Ketotifen fumarate affected this PGE2 production and reduced allergic inflammation with unknown antihistamine efficacy. PGs have an important role in the study of allergic inflammation and have potential importance in managing ocular allergic inflammation.
Acknowledgments
The authors thank Dr Takahashi for the diagnosis of atopic dermatitis. They also thank Ms Emi Akagawa and Ms Ayako Shibata for their technical assistance.
References
Footnotes
Contributors All authors reviewed, edited and approved the final version of the manuscript before submission.
Funding This study was supported financially by Santen Pharmaceutical Corporation, and in part by Kirin Brewery Company.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics review board of Tsurumi University School of Dental Medicine (Kanagawa, Japan; IRB number: 1111).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available.