Article Text
Abstract
Objective To study the influence of glycosylated haemoglobin (HbA1c) on response of bevacizumab in patients with diabetic macular oedema.
Methods and Analysis A total of 37 eyes of 37 patients with vision loss due to diabetic macular oedema treated with bevacizumab were included in this study. Participants received monthly intravitreal bevacizumab (0.05 mL/1.25 mg) for 3 months.
Results There were 17 patients with baseline HbA1c ≤7% (<53mmol/mol) and 20 patients with baseline HbA1c >7% (>53mmol/mol) treated with bevacizumab included in the study. The mean improvement in visual acuity at 3 months was 0.50 logMAR in HbA1c ≤7%(<53mmol/mol) group and 0.33 logMAR in HbA1c >7%(>53mmol/mol) group (95% CI,-0.05-0.38; p=0.13). The mean central macular thickness (CMT) reduction was −229.76 µm in patients with a baseline HbA1c ≤7% (<53 mmol/mol) and −145.20 µm in patients with HbA1c of >7% (>53mmol/mol) (95% CI,12.98-156.14; p=0.022).
Conclusion Our study suggests that baseline glycaemic control can affect the treatment outcome of intravitreal bevacizumab in the management of diabetic macular oedema and the response was found to be better in patients with good glycaemic control (low HbA1c).
- macula
- retina
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key message box
What is already known about the subject?
The relation between glycaemic control and diabetic macular oedema (DME) is well known.
One of the main treatment modalities for DME is anti-vascular endothelial growth factor (VEGF).
However, what factors affect the treatment response to anti-VEGF is still inconclusive.
What are the new findings
Early intervention with Anti-VEGFs could yield better results in diabetic macular oedema.
Visual and anatomic outcomes may or may not correlate each other and depends on multiple factors like chronicity of oedema,presence of macular ischaemia,etc.
How might these results change the focus of research or clinical practice?
Baseline HbA1c can be used as a predictive marker in treatment of DME with Anti-VEGFs.
The results can be helpful during patient counselling regarding the probable treatment outcome and importance of maintaing glycemic control throughout to attain the optimal results.
Introduction
Diabetic macular oedema (DME) is a major cause of vision loss in patients with diabetes mellitus (DM)1 and the prevalence increases from 0%–3% in individuals with recent diagnosis of DM to 29% in those with DM for more than 20 years.2 Based on the results of Early Treatment Diabetic Retinopathy Study (ETDRS) group, focal/grid laser photocoagulation was considered to be the gold standard treatment for management of DME.3 However, 12% of the treated eyes still lost 15 or more ETDRS letters at the 3-year follow-up interval.4 With the demonstration of role of vascular endothelial growth factor (VEGF) in the pathogenesis of DME, its inhibition seemed to be a good therapeutic option.5 More recently, the standard of care for DME is shifting toward intravitreal VEGF inhibitors.
Intravitreal inhibitors VEGF-A have become the first-line treatment for patients with DME, based on data from several large, prospective, randomised phase 3 clinical trials, including Diabetic Retinopathy Clinical Research Network protocol I,6 RIDE/RISE,7 8 RESOLVE,9 RESTORE10 VIVID and VISTA.11 Multiple studies have shown the efficacy of VEGF inhibitors in the treatment of DME and have suggested both anatomical and functional improvement in outcome.7 12 13 Despite these promising outcomes, benefit of the aforementioned therapies is found to be variable among patients. Previous studies have demonstrated the importance of systemic factors for progression of diabetic retinopathy and vision loss.
Several local and systemic factors, including duration of DM, serum glycosylated haemoglobin A1c (HbA1c) level, blood pressure control, presence of nephropathy and serum lipids, may play an important role not only in the presence of DME but also in its responsiveness to therapy. The relationship between HbA1c level and risk of microvascular complications is well established14 and HbA1c levels of 8%(64 mmol/mol)or more are associated with a greater risk of DME.15
In a retrospective study of 124 patients, Matsuda et al16 showed that in patients with HbA1c values of more than 7%(53mmol/mol), less robust anti-VEGF-mediated improvements in best-corrected visual acuity (BCVA) and central subfield macular thickness were achieved than in patients with HbA1c levels of 7%(53mmol/mol) or less. Similar findings from 65 patients were reported by Ozturk et al17 wherein reduction in DME with anti-VEGF therapy was correlated negatively with HbA1c level.
The purpose of this study was to evaluate the influence of glycaemic control in responsive to intravitreal anti-VEGF therapy, bevacizumab. We investigated the prognostic effect of HbA1c levels on the resolution of oedema and the visual and anatomical outcomes following therapy in patients with DME.
Materials and methods
Patients with centre-involving DME were included in the study after taking informed/written consent. Exclusion criteria were patients previously treated with any other intravitreal injection or focal/grid laser, loss of vision or macular oedema due to reason other than diabetes.
Preinjection clinical variables included best-corrected Snellen visual acuity (converted to logMAR value for statistical analysis), dilated fundus examination, intraocular pressure (IOP) and central macular thickness (CMT) (OCT SPECTRALIS-HEIDELBERG ENGINEERING). Serum HbA1c. Patients participating in the study were treated with 0.05 mL/1.25 mg intravitreal bevacizumab injection monthly apart for 3 months and were followed at 4, 8 and 12 weeks. On every follow-up, detailed examinations were done including BCVA, IOP, dilated fundus examination and OCT macula.
Baseline HbA1c analysis
To investigate the influence of baseline HbA1c on treatment outcomes, patients were separated into two subgroups on the basis of baseline HbA1c <7%(<53mmol/mol) or >7%(>53mmol/mol). This criterion was chosen on the basis of 2014 American Diabetes Association position statement on the generally accepted threshold of diabetic control.18
Anti-VEGF injection technique
Intravitreal injections were performed in the operating room under sterile conditions. Bevacizumab (Avastin; Roche, Manheim, Germany) 1.25 mg in 0.05 mL was injected into the vitreous cavity using a 30 G needle through pars plana, 3.5–4.0 mm posterior to limbus.
Data processing and statistical analysis
Data were statistically described in terms of range, mean±SD, frequencies (number of cases) and percentages when appropriate. The mean change of logMAR visual acuity and CMT from baseline were assessed using paired t-test. In addition, to analyse treatment effects in different HbA1c subgroups, the study population was divided into groups with <7%(<53mmol/mol) and (>7%53mmol/mol) HbA1c levels. Independent t-test was used to compare the mean change from baseline BCVA and CMT at 3 months between HbA1c subgroups. P value less than 0.05 was considered the level of significance for all calculations. All statistical calculations were done using computer programs Microsoft Excel 2016 and SPSS V.23 for Microsoft Windows.
Results
Baseline characteristics
A total of 37 eyes of 37 patients with type 2 DM were included in the study among which 68% were males and 32% were females with a mean age of 55.62±9.02 years (range 36–80 years). The mean duration of diabetes was 11.11±5.6 years (range 1–25 years). Most of the patients in our study had severe non-proliferative diabetic retinopathy (NPDR) (56.8%) followed by moderate NPDR (32.4%). Diffuse spongiform pattern of oedema was the most common (45.9%) pattern seen in OCT in our study. Cystoid oedema was seen in 37.8% of the cases. About 54.1% had HbA1c >7% (>53mmol/mol)and the rest (45.9%) had HbA1c <7% (<53mmol/mol)
Anatomical and functional outcomes
At baseline, the mean logMAR BCVA was 0.85±0.36 (range 0.2–1.8) and improved to 0.44±0.22 (range 0–1.00, p<0.001) at 3 months after three injections of bevacizumab. The mean baseline CMT was 498.19±106.42 µm (range 296–780 µm) and decreased to 314.13±70.77 µm (range 245–622 µm, p<0.001) at 3 months (table 1).
Mean and SD of best-corrected visual acuity (BCVA) and central macular thickness (CMT) at initial and final examination
HbA1c and its relation to anatomical and functional outcomes following bevacizumab
To study the treatment response in between subgroups of HbA1c, we divided the patients into two groups HbA1c <7%(<53mmol/mol) and HbA1c >7%(>53mmol/mol). Anatomically, mean change in CMT following bevacizumab in HbA1c <7%(<53mmol/mol) group was 229.76 μm and 145.20 μm in HbA1c >7%(>53mmol/mol) group (figure 1). The mean difference in CMT reduction between the two groups was 84.56 μm and was statistically significant (p=0.022).
Line graph showing mean central macular thickness (CMT) after each injection in two groups (glycosylated haemoglobin (HbA1c) <7% and HbA1c >7%).
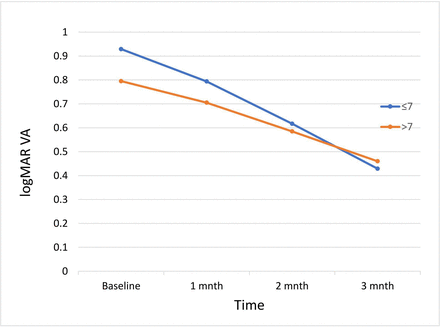
The mean change in logMAR BCVA was 0.50 in HbA1c <7% (<53mmol/mol)and 0.33 in HbA1c >7% (>53mmol/mol)(figure 2). The mean difference in change in logMAR BCVA was 0.16 and was not statistically significant (p=0.13).
Line graph showing mean logMAR visual acuity (VA) after each injection in two groups (HbA1c <7% and HbA1c >7%).
Discussion
There are several studies highlighting the importance of glycaemic control on the progression of diabetic retinopathy.1 15 19 However, only few studies have assessed the role of glycaemic control in treatment response to VEGF inhibition.16 17 20 21
Ozturk et al reported the impact of glucose regulation in DME treatment. In that report, more optimal serum HbA1c levels correlated with increased reduction in CMT following a single intravitreal injection.17 In our study also, we had increased reduction of CMT (229.76 μm) in group with HbA1c <7%(53mmol/mol) compared with 145.20 μm in group with HbA1c >7%(>53mmol/mol) and the difference was statistically significant (p<0.022). The average CMT difference was 84.56 μm more in groups with lower HbA1c than in the group with higher HbA1c.
In a small prospective analysis of 38 patients by Warid et al,22 greater proportion of patients with HbA1c <7%(<53mmol/mol)gained 2 lines of VA compared with those with HbA1c >7%(>53mmol/mol), suggesting that poorer glycaemic control may lead to worse visual outcomes.
By contrast, in a prospective study of 52 patients, Macky and Mahgoub20 reported that there was no difference in the 6-month VA or CMT between patients with baseline HbA1c <7%(<53mmol/mol) or >7%(>53mmol/mol)treated with three injections of bevacizumab plus laser. However, lower HbA1c appeared to be correlated with better visual acuity and lower CMT values at baseline.
In a retrospective analysis of 124 patients treated with bevacizumab DME over 12 months, Matsuda et al16 demonstrated that patients with a baseline HbA1c <7%(<53mmol/mol) had better VA (20/43) at 1 year compared with patients with a baseline HbA1c >7%(>53mmol/mol) (20/62). However, there was no significant difference in the final CMT at 1 year between the two groups; that is, both groups had significant reductions in CMT after treatment regardless of their glycaemic control.
In a post hoc analysis by Bansal et al,21 the patients treated with monthly intravitreal ranibizumab had improvement in VA, reduction in CMT and improvement in DR severity score independent of their baseline HbA1c or change in HbA1c. There was no significant differences in the 36-month vision, change in vision or 36-month CMT between patients with baseline HbA1c <7%(<53mmol/mol) and >7%(>53mmol/mol) or between patients stratified by quartiles of baseline HbA1c.
In our study, the patients with more optimal DM control (HbA1c <7%) had a mean improvement in logMAR VA of 0.50 at 3 months. Those patients with less optimal DM control at baseline (HbA1c >7%) also had an improvement (0.33 logMAR) but the change was less marked in comparison to the HbA1c <7% group. The difference in the final BCVA between the two groups was not statistically significant (p=0.13)
Although a marked reduction of macular oedema was found in both the subgroups of patients with HbA1c <7% (<53mmol/mol)and HbA1c >7%(>53mmol/mol), the visual outcome did not correlate with improvement in CMT.
The quantitative assessment of macular thickness using OCT is clinically useful, but macular thickness is just one of several variables affecting visual outcomes.23 The discordance between visual and anatomical outcome in our study may be related to factors like chronic nature of oedema, macular ischaemia and so on.
As it is to our knowledge, the irreversible photoreceptor damage due to long-standing macular oedema may limit a correlated increase in visual acuity.24 So, if there is photoreceptor cell damage, anatomical improvement may not contribute to visual improvement.
Macular ischaemia is also known to be associated with poor visual outcome in patients with diabetes regardless of treatment.25 Since fluorescein angiographies were not done in all the cases, information regarding macular perfusion was limited. Other factors like presence of epiretinal membrane, posterior hyaloid traction may also contribute to the poor visual outcome.
However, the limited initial BCVA response does not entirely preclude the possibility of better BCVA response in the long term. The follow-up duration in our study was relatively short, and it is known from large clinical trials that stabilisation of VA takes time and it can improve even after the loading dose of three injections.
There could be several reasons for the differences in our results and those of other studies. The duration of follow-up, choice of anti-VEGF injection, number of injections all can influence the final outcome. Also, as we know, DME is a complex condition and the improvement or worsening of DME may depend not only on HbA1c or VEGF alone but also on multiple systemic and local factors, such as blood pressure, cholesterol, obesity, which all can confound the final results.
In conclusion, intravitreal bevacizumab treatment resulted in an improvement in visual acuity and decrease in macular thickness in DME. Maximum benefit from the treatment may be attained by strict glucose regulation. Glycaemic control influences the treatment outcome and may be responsible in part for the different response among patients with the same DME treatment.
The functional and anatomical outcomes are usually correlated but the changes in anatomy alone do not explain the final functional outcome specially in cases with poor glycaemic control who may have chronic macular oedema. Therefore, every early intervention for DME without ischaemia may help to preserve the visual potential by inhibiting further degeneration of photoreceptors. Thus, there is a critical role of a multidisciplinary approach to the patient with DME, in particular for coordination between the endocrinologist and the treating vitreoretinal specialist
References
Footnotes
Contributors SS, SNJ, PK conceptualised the study. SS contributed in data collection, data analysis and preparation of manuscript. SNJ and PK reviewed and edited the manuscript. All authors approved the final manuscript. All authors had complete access to the study data.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval Ethics approval was taken from the institutional review board of Institute of Medicine, Tribhuvan University.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon request.