Article Text
Abstract
Objective To compare the performance and safety in cataract surgery of two ophthalmic viscoelastic devices (OVDs), each having separate dispersive and cohesive characteristics and different concentrations.
Methods and analysis In this prospective, randomised, controlled clinical study, one eye of each patient was injected with OVD1 (Viscopack14) or OVD2 (DuoVisc) during phacoemulsification and intraocular lens implantation. Endothelial cell count, intraocular pressure (IOP), central corneal thickness (CCT), intraocular inflammation and corrected distance visual acuity (CDVA) were compared 3 months postoperatively.
Results The study enrolled 127 patients. Randomisation assigned 50 eyes of as many patients to each arm of the study. The postoperative mean endothelial cell loss was 14.4% and 7.1% from baseline in the OVD1 and OVD2 groups, respectively (p=0.08). The incidence of IOP spikes at 2 hours was 0% and 8%, respectively (p=0.02). There were intergroup differences in postoperative IOP values, the OVD2 group showed significantly higher values at all of the follow-up visits starting from the 24 hours examination (p<0.05). There was no statistically significant difference in the CCT, intraocular inflammation and CDVA at the end of follow-up.
Conclusion Both OVDs showed similar clinical performances and were equally effective during cataract surgery. Viscopack14 showed more corneal endothelial cell reduction, while DuoVisc had more occurrences of IOP values and spikes. Future studies are mandatory to support these preliminary results.
- ophthalmic viscoelastic device
- cataract surgery
- endothelial cell count
- intraocular pressure
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Safety and effectiveness of DuoVisc have been tested in a series of controlled clinical trials.
What are the new findings?
Viscopack14 is as efficient and safe during cataract removal by phacoemulsification as DuoVisc.
How might these results change the focus of research or clinical practice?
Our results indicate that DuoVisc might be favoured in patients with cornea guttata or low endothelial cell count. On the other hand, a lower incidence of IOP spikes with Viscopack14 might make its use safer in patients with glaucoma. Future studies should test these hypotheses in high-risk patient populations, such as those affected by endothelial corneal dysfunction or glaucoma/hypertension.
Introduction
Ophthalmic viscoelastic devices (OVDs) are commonly classified into two main categories depending on their rheological properties: dispersive and cohesive.1 Dispersive OVDs have lower molecular weight and shorter molecular chains that cause better adherence to the corneal endothelial cells resulting in greater protection against fluid turbulence and lens fragments during phacoemulsification.2 Cohesive OVDs have high molecular weight and long molecular chains that promote spaces maintenance.3 Nevertheless, both OVDs have some limitations. The short molecular chains make the dispersive OVD mass more prone to be divided and, therefore, more difficult to remove, causing more postoperative intraocular pressure (IOP) spikes.1 In contrast, cohesive OVDs are quicker to be removed but offer lower protection to the corneal endothelium.1 In 1999, Arshinoff described the soft-shell technique, a surgical technique with a dispersive and a cohesive OVD simultaneously. The dispersive OVD is placed first to coat the corneal endothelium, and then the cohesive OVD is injected centrally to flatten the anterior lens capsule, deepen the anterior chamber, force the dispersive OVD towards the cornea and make of the capsulorhexis.1 To this purpose, DuoVisc (Alcon Laboratories, Fort Worth, Texas, USA) was devised as a packet with two syringes, each containing a dispersive combination of sodium hyaluronate (NaHA) and chondroitin sulfate (CS) (Viscoat), and a cohesive NaHA solution (ProVisc). Safety and effectiveness of DuoVisc have been tested in a series of controlled clinical trials.4–6 An experimental model suggested the optimised concentration of CS provides Viscoat three negative charges for protection versus one for NaHA-containing products, for greater binding to the corneal endothelium.2
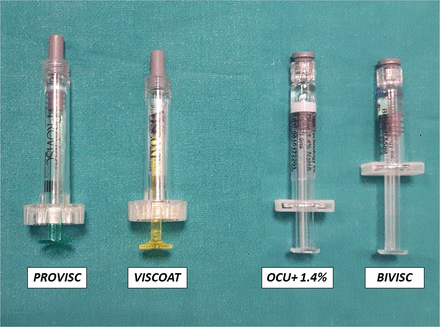
The aim of this study was to compare the clinical performance and safety of a new dual-OVD, the Viscopack14 (assembled by SGM Srl and distributed by Diemme Dispositivi Medici, Triulzi, Italy), with DuoVisc (Alcon Laboratoires, Fort Worth, Texas, USA) used as a comparator (figure 1). Viscopack14 combines a dispersive (BiVisc, CIMA Technology, Pittsburgh, Pennsylvania, USA) and a cohesive OVD (Ocu+, CIMA Technology), and it is similar to DuoVisc but with different concentrations in the attempt to provide a clearer visibility and a better space maintenance during surgery, without losing performance in endothelial cell coating and inducing postoperative IOP spikes.
DuoVisc viscoelastic system consists of Viscoat and ProVisc ophthalmic viscosurgical device (OVD). Viscopack14 consists of Ocu+ and BiVisc OVD.
Materials and methods
This was a prospective, randomised, double-blind, controlled clinical study. The principles of Good Clinical Practice were adhered to in accordance with the Declaration of Helsinki.
Patient enrolment and randomisation
The study included patients having cataract surgery and willing to adhere to the study protocol. All patients had a full ophthalmological examination, including refractive status, near and distance visual acuity (VA), slit-lamp examination, tonometry, funduscopy, biometry, corneal topography and optical coherence tomography.
Inclusion criteria were lens opacity causing a reduction in visual quality. Exclusion criteria were inability to cooperate, difficulties comprehending written or spoken Italian language, endothelial cell count (ECC) below 1200 cells/mm2, glaucoma and/or IOP higher than 24 mm Hg, hypermature cataract, ocular comorbidity that may hamper postoperative VA, previous refractive surgery, pseudoexfoliation and/or zonular fibre weakness, optical biometry impractical because of dense cataract, and axial lengths of 20.00 mm or shorter or 26.00 mm or longer.
Eligible patients were identified at their preoperative assessment and provided with an explanation of the study and its aims. The patients were provided with detailed study information, designed to be readily comprehensible to a non-expert reader. Written consent to participate in the study was obtained before surgery.
After enrolment, patients were randomly assigned to receive either Viscopack14 (OVD1; Group 1) or DuoVisc (OVD2; Group 2). Only one eye of each patient was evaluated in the study. Randomisation was performed using Minim, a free minimisation programme.7
Ophthalmic viscosurgical devices
Viscopack14 is available in two individual syringes, each containing a dispersive (BiVisc) syringe and a cohesive (Ocu+) syringe OVD (table 1 and figure 1). BiVisc is a clear, sterile, non-pyrogenic viscoelastic preparation of high molecular weight NaHA (sodium hyaluronate 2%) and highly purified CS (chondroitin sulfate 3%) dissolved in a physiological buffer. The solution is supplied in a single-use disposable Luer-lock glass syringe and accompanied with a 27-gauge (27G) cannula. Ocu+ is a clear, sterile, non-pyrogenic viscoelastic preparation of high molecular weight NaHA (sodium hyaluronate 1.4%) supplied in a single-use disposable Luer-lock glass syringe and accompanied with a 27G cannula. DuoVisc also comes in two syringes in one box, each containing a dispersive (Viscoat) and a cohesive (ProVisc) OVD (table 1 and figure 1). Viscoat is a sterile, non-pyrogenic, viscoelastic solution of highly purified, non-inflammatory medium molecular weight CS (chondroitin sulfate 4%) and NaHA (sodium hyaluronate 3%). ProVisc is a sterile, non-pyrogenic, high molecular weight, non-inflammatory highly purified fraction of NaHA (sodium hyaluronate 1%), dissolved in physiological sodium chloride phosphate buffer. Both solutions are supplied in a disposable syringe packaged with a sterile 27G, disposable, blunt-tip cannula and cannula-locking ring.
Characteristics of the two OVDs (data extracted from the instructions for use provided by the manufactures)
Surgical technique
All surgeries were performed by the same surgeon (AS) using a standardised technique. A 2.2 mm limbal self-sealing clear corneal incision was created. According to the randomisation, the anterior chamber was filled with BiVisc or Viscoat. A continuous curvilinear capsulorhexis was created. Hydrodissection was followed by phacoemulsification (Infiniti Vision System OZil, Alcon Laboratories) using a bimanual cracking technique and coaxial Irrigation/Aspiration (I/A). Standardised parameters were used during phacoemulsification and I/A as follows: flow, 30 mL/min and vacuum, 425 mm Hg for chopping and fragment removal, and 400 mm Hg for I/A, both in proportional mode. For IOL implantation, the capsular bag and the anterior chamber were filled with Ocu+ or ProVisc. All IOLs were implanted in the capsular bag using the manufacturer’s recommended IOL loading and injection technique. After IOL implantation, the OVD was carefully removed from the anterior chamber and capsular bag by I/A. Care was taken to aspirate all OVD from the anterior chamber and bag by slightly tilting the IOL and positioning the I/A tip behind. Postoperative treatment consisted of 0.1% dexamethasone/0.3% netilmicin (Netildex, Sifi, Catania, Italy) eye drops for 2 weeks followed by bromfenac 0.9% (Yellox, Bausch & Lomb, Rochester, New York, USA) eye drops for 2 weeks.
Outcomes and assessment
All patients were examined preoperatively and postoperatively in accordance with routine institutional clinical care policies for patients having cataract surgery. An ophthalmologist (MG), an ophthalmic assistant (GR and IT) and a trained certified optometrist (SP) assessed primary and secondary outcomes up to 3 months after surgery.
The primary outcome measure was ECC. Secondary outcomes were IOP, central corneal thickness (CCT), intraocular inflammation and corrected distance VA (CDVA).
ECC was assessed with a non-contact specular microscopy and its integrated software (Noncon Robo SP6000, Konan Medical, Irvine, CA, USA) at the inclusion visit for baseline value and at 90 days after the surgery to assess the mean cell density loss over time. The mean of three separate measurements of the central cornea was used as the ECC value (cells per 1 mm2). For each measurement, the examiner selected a photo with good-quality cell borders and marked at least 100 contiguous cells, to obtain an analysis of at least 50 cells. If the examiner was unable to obtain accurate measurements from the first selected photo, the second photo was used for the calculation.
The IOP was measured with an automatic non-contact tono/pachymeter and its software (NT-530P, Nidek Co., Bunkyo City, Hongo, Tokyo, Japan) at the inclusion visit for baseline value, at 2 and 24 hours after surgery, and then after 7, 45 and 90 days. The tono/pachymeter measures the IOP three times providing a reliable value representing the compensated IOP based on the CCT.8 At each examination, the incidence of IOP peaks of 24 mm Hg or higher was recorded. Cases of elevated IOP of 30 mm Hg or higher would be treated with local and/or systemic anti-glaucoma medication(s) or with paracentesis for pressure relief at the discretion of the examiner.
CCT was measured using the optical low coherence reflectometry (OLCR) ocular biometry device at the inclusion visit, at 24 hours after surgery, and then after 7, 45 and 90 days. The OLCR ocular biometer simultaneously provides up to nine biometric assessments in a single measurement. With regard to the measurements of CCT, it has been shown to be comparable with ultrasound pachymetry.9
Intraocular inflammation was evaluated subjectively by slit-lamp examination at the inclusion visit and at 24 hours after surgery, and then at 7, 45 and 90 days. The inflammation was evaluated at the slit lamp according to the Standardization of Uveitis Nomenclature for reporting clinical data.10 Inflammatory cells (aqueous cells) and the optical density of protein (aqueous flare) in the anterior chamber were reported independently on a scale from 0 to +4 using a field size of 1 × 1 mm slit beam. The limit of clinical relevance in this study was considered to be +1 in terms of either cells or flare. When the inflammation was graded as +3 or more at any visit, patients would have been given extra steroid eye drops regardless of their randomisation group. CDVA was evaluated at the inclusion visit, and then at 45 and 90 days after surgery. CDVA was measured with the logarithm of minimum angle of resolution notation at 100% contrast using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts (ETDRS Standardized Viewer Model No. ESV3000), under photopic conditions (85 candelas/m2) at 3 m.
The cumulative dissipated energy (CDE) was recorded prospectively on clinical record forms at the end of surgery. The value is displayed automatically on the interface of the Infiniti Vision System and is measured in per cent-seconds. CDE is the total energy dissipated at the wound site in foot position 3, including a combination of torsional and longitudinal ultrasound energies. The CDE values for the two groups were compared with control, a risk for bias caused by intraoperative conditions.
Masking
This was a double-blind study. The OVD assignments were individually enveloped and numbered. Each patient was then assigned an envelope, opened at the beginning of surgery. OVD type was recorded in patient surgical files. Examiners performing outcome assessments did not have access to surgical files and were blinded to which OVD was used. Statisticians were also masked to OVD identity until analysis of the data.
Sample size
Calculation of the required sample size was based on the primary outcome parameter of ECC. A previously published study with the DuoVisc used in this study found a mean cell reduction of 9.6%.6 Considering a SD of 0.2, a difference of 5% was assumed to be clinically significant. Based on these assumptions, an alpha of 0.01 and a power of 0.9, it was calculated that 45 eyes were required in each group. Assuming a dropout rate of 10%, this resulted in a total requirement of 99 eyes. A total sample size of 100 was planned.
Statistical analyses
Statistical analyses were performed using STATA V.14.0 for Windows. The level of statistical significance was set at p<0.05. All tests were two-tailed. Intergroup comparisons of baseline characteristics were performed using the χ2 test and one-way analysis of variance (ANOVA) for categorical and continuous variables, respectively. One-way ANOVA was used to compare the primary and secondary outcomes at 3 months after surgery. Post hoc analysis was adjusted using the Bonferroni correction. Furthermore, ANOVA for repeated measures was used to examine the within and between-group effects on the dependent variable.
Results
One eye of each patient was evaluated in the study. A total of 127 patients (127 eyes) were assessed for study eligibility between September 2017 and February 2018 and the screening was stopped at the 100th enrolled patient. Randomisation assigned 50 eyes of as many patients to each arm of the study. There were no statistically significant intragroup differences in baseline characteristics for eyes assigned to any arm of the study (table 2). All recruited patients completed the study after enrolment. There were no dropouts. Unexpected eye examination findings and surgical complications were not observed. The mean CDE value recorded during the surgeries was 8.2 s±4.1 (SD) and 8.3 s±4.3 (SD) in Group 1 and Group 2, respectively (p=0.6).
Baseline characteristics of the groups
Primary outcome measure
In Group 1, the mean postoperative endothelial cell loss at 3 months was −323.0±651.1 cells/mm2, corresponding to a mean cell density reduction of 14.4% from baseline. In Group 2, the mean postoperative endothelial cell loss at 3 months was −163.0±486.9 cells/mm2, corresponding to a mean cell density reduction of 7.1% from baseline. No statistically significant difference in the mean ECC between the two groups (p=0.08) was found. However, Group 1 showed a postoperative ECC that was significantly lower than the baseline values (p=0.00)(table 3; figure 2).
Postoperative data of the OVD1 and OVD2 groups
Box plot of endothelial cells count (ECC) at baseline and after 90 days to surgery. OVD1 showed a postoperative ECC that was statistically significantly lower than the baseline values (p=0.00). OVD, ophthalmic viscoelastic device.
Secondary outcome measures
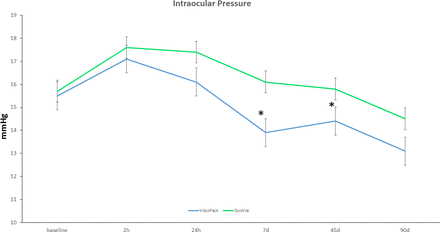
The preoperative and postoperative mean IOP measurements are presented in tables 2 and 3. Two hours after the surgery, we observed a significant increase in mean IOP from the baseline value in both groups (p=0.00), followed by a return to preoperative values at 24 hours postoperatively for Group 1 and at the 7-day measurement for Group 2. At the 90-day visit, the IOP in both groups was lower than preoperatively (p<0.01). There were intergroup differences in postoperative IOP values; the Group 2 showed significantly higher values at any of the follow-up visits except for the 2-hour examination (p<0.05) (figure 3). The incidence of IOP peaks (24 mm Hg or higher) at 2 hours from surgery was 0 patients in Group 1 and 4 patients (8%) in Group 2 (p=0.02). Twenty-four hours after the surgery, two patients (4%) in Group 1 and five patients (10%) in Group 2 had an IOP peak (p=0.01), out of which one reached a value of 30 mm Hg that was resolved with appropriate medication (two tablets of acetazolamide 250 mg). All IOP peaks in Group 2 were resolved at 1 week from surgery. No IOP peaks were observed in Group 1 in all visits after surgery.
Changes in mean intraocular pressure (IOP) before and after 90 days to surgery. Two hours after the surgery, a significant increase in mean IOP from the baseline value was observed in both groups (p=0.00), followed by a return to preoperative values 1 day postoperatively for OVD1 and at the 7-day measurement for OVD2. At the 90-day visit, the IOP in both groups remained lower than preoperatively (p<0.01). *P value<0.05 was calculated with ANOVA. 2h, 2 hours; 7d, 7 days; 24h, 24 hours; 45d, 45 days; 90d, 90 days; ANOVA, analysis of variance; OVD, ophthalmic viscoelastic device.
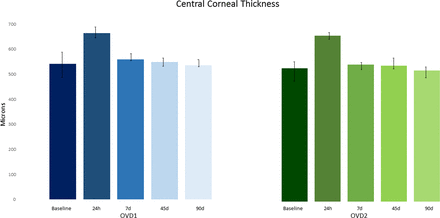
In Group 1, the mean postoperative CCT variation at 24 hours was 58.3±9.1 (SD) microns, corresponding to a mean increase of 10.8% from baseline (p=0.02). In Group 2, the mean postoperative CCT variation at 24 hours was 58.6±7.3 (SD) microns, corresponding to a mean increase of 10.6% from baseline (p=0.03). However, there was no statistically significant difference in the CCT from baseline to the 90-day examination between the two groups (table 3; figure 4).
Changes in central corneal thickness (CCT) before and after 90 days to surgery. There was no statistically significant difference in the CCT from baseline to the postoperative examination between OVD1 and OVD2. 7d, 7 days; 24h, 24 hours; 45d, 45 days; 90d, 90 days; OVD, ophthalmic viscoelastic device.
At 24 hours postoperatively, mild inflammation in both groups for all criteria (cells and flare) (p=0.0001) was observed. However, at 7, 45 and 90 days postoperatively, the inflammation was close to baseline. There was no statistically significant difference between the two groups at any follow-up visit for any criteria (p=0.83) (table 3).
No statistically significant differences were found in CDVA between the two groups at any follow-up. In both groups, the CDVA was found to significantly improve postoperatively compared with the baseline values (p=0.00) (table 3).
Discussion
The OVD plays a central role in phacoemulsification to assist intraocular surgical manoeuvres and to reduce damage to the intraocular structures. Different products have been devised within each OVD class, including dual-OVD. To the best of our knowledge, the Viscopack14 has not been previously evaluated in a prospective clinical study. We compared it with DuoVisc, the first and widely used dual-OVD of the same class, in conventional coaxial phaco-chop cataract surgery. The study focused exclusively on objective parameters, excluding subjective surgeons’ appreciations. Both the two groups were operated on by the same surgeon and were comparable in baseline characteristics, as well as in ultrasound (US) energy (CDE) employed during the surgery.
The ability to protect corneal endothelial cells is a primary OVD goal since the corneal endothelium actively contributes to maintain optical transparency.11 12 At 3 months, no significant intergroup differences were found in postoperative ECC values (p=0.08). DuoVisc caused a mean endothelial cell loss of 7.1%, a value comparable to that reported by Auffarth et al (9.6%) in a recent multicentre study, where DuoVisc was tested against Twinvisc (Carl Zeiss Meditec AG, Jena, Germany).6 A similar DuoVisc cell loss also resulted in meta-analysis by Yu et al of randomised controlled trials, reporting an endothelial cell loss rate of 2.4%–12.3% at 3 months postoperatively, after conventional coaxial phacoemulsification using different OVDs.13 In contrast, in our study, Group 1 showed a postoperative ECC significantly lower than its baseline values, corresponding to a mean cell density reduction of 14.4%. In the cited Auffarth study, Twinvisc showed a mean density loss of 11.7%, higher than DuoVisc. These results are similar for our groups, including non-significance decrease of the ECC decrease.
Our results confirm that DuoVisc is more protective to the ECC than the Viscopack14. We may hypothesise that this is due to DuoVisc having a greater concentration in NaHA but most of all in CS compared with Viscopack14 (3% NaHA plus 4% CS vs 2% NaHA plus 3% CS), since CS makes OVDs more dispersive, enhancing their negative surface charges and increasing binding to the corneal endothelium.2
IOP elevation in the early postop days is attributed to residual intraocular OVD gradually released into the aqueous and mechanically obstructing the trabecular outflow pathway.14 The IOP elevation is ordinarily transient and well-tolerated, though prolonged spikes of hypertension may lead to pain, corneal oedema and optic nerve damage. Since it is more challenging to entirely remove the dispersive component of the dual-OVDs, mainly in anterior chamber (AC) recesses and from endothelium, the effect of the residual material on the postop IOP is one of the critical safety parameters.5 6 15 In our study, the OVDs were removed with care from the anterior chamber, as well as from behind the IOL, at the end of the surgery. Nevertheless, a significant increase in mean IOP from the baseline value was observed in both groups at 2 hours (p=0.00), though mostly within physiological values, followed by a return to preoperative values 1 day postoperatively for Group 1 and at 7 days for Group 2. For Viscopack14, our findings were consistent with those reported in the literature of an early postoperative IOP elevation and a return to baseline values after approximately 24 hours.5 6 15 The Auffarth et al study found a non-significant increase in both groups at 6 hours, but peaking above 24 mm Hg in 16.8% and 25.2%, respectively. This same trend was seen in our study, where the elevation of the IOP in the DuoVisc group was more persistent in time, requiring more days to recover. Four patients (8%) were also observed with IOP spikes versus zero patients in the OVD1 group (p=0.02). These results are very interesting but they could suggest that there was an intergroup difference in trabecular or outflow function of the patient’s population. For better clarify the trend of IOP provided by the two OVDs used in this study, the authors believe more useful including, in a future study, patients from high-risk populations such those affected by glaucoma or ocular hypertension.
The postop CCT increase may reflect the effect of the surgery on the endothelial stress and function, caused by US energy and turbulence of the irrigation solution, as well as uncontrolled bouncing nuclear fragments.16 17 The evolution of the mean CCT followed an already described pattern with a peak observed 1 day postoperatively and a recovery phase, regaining normal values 2 weeks after surgery.18 In our study, 1 day postoperatively, CCT increased of 10.8% and 10.6% in OVD1 and OVD2 groups, respectively, from baseline was observed (p=0.03), and it was consistent with findings previously reported using sequentially dispersive and cohesive OVDs.6 18 19 There was no statistically significant difference in the mean CCT between the two groups at any of the postop controls.18 Such findings indicate that both OVDs provided equivalent mechanical protection and a good and uniform quality of surgery. We did not find a correlation between surgical trauma and endothelial cell loss at 3 months like Lundberg et al,20 since there was no difference in CCT increase and inflammation signs in the first postop controls.
Measuring the postoperative anterior chamber inflammatory response assessed the safety of the two OVDs during cataract surgery. In our study, there was evidence of mild inflammation in both groups at 24 hours. However, at 7, 45 and 90 days postoperatively, the inflammation level was very close to baseline values. These findings confirm that the inflammation level caused by the surgery was identical in the two groups and that both were well-tolerated.
There are limitations to the current study. Only otherwise healthy (less vulnerable) eyes were included inducing a selection bias due to a sample that does not accurately reflect the target population. The overall population for which the measure of effects was calculated didn’t include patients affected by endothelial corneal dysfunction or glaucoma, generally present in a cataract session. Including such patients would have strengthened the paper. However, the authors wanted to prevent factors not properly related to cataract surgery from influencing the results of this preliminary study assessing the safety of Viscopack14.
In summary, our results suggest that Viscopack14 was as efficient and safe during cataract removal by phacoemulsification as DuoVisc. No clinically relevant differences were found between the two devices. However, in considerations of a lower incidence of IOP spikes with Viscopack14 might make its use safer in patients with glaucoma. Future studies should test this hypothesis in a high-risk patient population, such as glaucoma patients, as these current results may not be clinically significant in patients with healthy nerves and no glaucoma.
References
Footnotes
Contributors GM planned, conducted and reported the work described in the article. MG co-conducted the study. AS revised the manuscript. SP, GR and IT collected the data. AL analysed the data.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.