Article Text
Abstract
Objective We investigated the efficacy of additional topical betamethasone in persistent cystoid macular oedema (CMO) after carbonic anhydrase inhibitors (CAIs) therapy.
Methods and analysis This retrospective cohort study included 16 eyes of 10 patients with retinitis pigmentosa (RP). All patients were previously administered CAI for at least 3 months to treat CMO secondary to RP and lacking an effective reduction (≥11%) of central foveal thickness (CFT). We administered topical 0.1% betamethasone daily in each affected eye following a preceding course of the CAI medication as a first treatment. CMO was diagnosed using spectral-domain optical coherence tomography. CFT was regarded as the average of vertical and horizontal foveal thickness. Best-corrected visual acuity (BCVA) and intraocular pressure (IOP) were obtained from patient medical records. We compared the CFT and BCVA between baseline and the average of 1–3, 5–7, 10–14 and 16–20 months period.
Results In treatments with brinzolamide in 14 eyes, dorzolamide in 2 eyes and bromfenac in 2 eyes, CFT effectively decreased in 12 of 16 eyes (81%). CFT decreased significantly in 1–3 months (326±102 µm; n=16; P=0.029) and 5–7 months (297±102 µm; n=12; P=0.022) compared with baseline but not within 10–14 months (271±96 µm; n=9; P=0.485) or 16–20 months (281±134 µm; n=9; P=0.289). There were no significant intergroup differences in BCVA throughout the study. Betamethasone treatment was stopped in three patients because of IOP elevation.
Conclusion Our data suggested that additional betamethasone might improve treatments for persistent CMO. Topical steroids could be an alternative option for managing persistent CMO in RP.
- retina
- treatment medical
- drugs
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Cystoid macular oedema (CMO), a vision-threatening condition, is one of the most frequent complications in retinitis pigmentosa (RP). Although various treatment approaches using carbonic anhydrase inhibitors (CAIs) or steroids have been reported, and intravitreal or subtenon’s corticosteroids have shown efficacy in managing CMO, a less invasive topical steroid therapy has been studied in only one case report. The present study hence retrospectively investigated the efficacy of additional topical betamethasone in persistent CMO after CAI therapy in RP, representing specific treatments that have not been previously reported.
What are the new findings?
Adding 0.1% topical betamethasone administration with CAI treatment decreased central foveal thickness in 1–3 and 5–7 months period compared with baseline.
How might these results change the focus of research or clinical practice?
Although there were no previous studies that focused on additional treatments to persistent CMO in RP, adding topical steroid to previous CAI treatments may be an improvement for managing CMO in RP.
Introduction
Retinitis pigmentosa (RP) is an inherited disease affecting 1 in 3000–5000 people and is characterised by progressive degeneration of the photoreceptors (rods and cones) and/or the retinal pigment epithelium (RPE).1 Typical RP involves a rod–cone dystrophy in which the first symptom is night blindness followed by a progressive loss of the peripheral visual field while central vision is generally preserved for several decades.2 Cystoid macular oedema (CMO) is one of the common complications in RP and causes central vision impairment in such patients.3 The prevalence of CMO in RP is 11%–20% by fluorescein angiography (FA) and fundus examination yet diagnosed at 38%–49% when using optical coherence tomography (OCT).4 OCT provides morphological information capable of observing cross-sectional graphic image and different meridians around the fovea. FA examines morphological and circulatory changes in the retina by imaging fluorescein leakage and accumulation. OCT is more sensitive for identifying minor structural changes that would be detected by FA once the fluorescein reaction has manifested. Therefore, it was reported that OCT is more sensitive than FA for identifying CMO.5
Many treatments have been reported to show some efficacy in managing CMO in patients with RP, including oral or topical carbonic anhydrase inhibitors (CAIs), intravitreal and subtenon corticosteroids, antivascular endothelial growth factor agents and vitrectomy.6 Several previous studies have reported that CAI and corticosteroids showed effective improvement of CMO in RP.7 However, CAI and corticosteroids may not adequately demonstrate desired effects or may permit recurrence by single agent treatment.8–10 Few studies have focused on additional treatments to persistent CMO in RP. Moreover, previous studies of treatment administration mainly reported either oral or topical CAI and either intravitreal or subtenon steroids; however, topical steroids have been shown in one case report.11 If effective, topical steroids would be convenient and much easier for patient care. The present retrospective cohort study examines the efficacy of additional topical betamethasone in persistent CMO after CAI therapy in patients with RP.
Materials and methods
This retrospective cohort study included 16 eyes of 10 patients seen in the RP clinics at Kobe City Medical Center General Hospital, Kobe, Japan, from 2009 to 2016 inclusive. This study protocol was conducted in accordance with the tenets of the Declaration of Helsinki. We obtained the patients’ data from the Kobe City Medical Center General Hospital.
Diagnosis of RP was based on the patient’s history of night blindness, visual field construction, bone-spicule-like pigment clumping, presence of central perimacular hyperautofluorescent rings and full-field electroretinogram testing suggestive of rod–cone dystrophy.
Inclusion criteria
All patients were previously given the CAI alone or CAI and non-steroidal anti-inflammatory drugs (NSAIDs) for at least 3 months to treat CMO secondary to RP and lacking an effective reduction (≥11%) of central foveal thickness (CFT). From this group, we administered additional topical 0.1% betamethasone three or four times daily in each affected eye after the CAI alone or CAI and NSAIDs medication as a first treatment.
Exclusion criteria
Patients were excluded if they had other causes such as epiretinal membrane, vitreous traction, diabetes or uveitis, or if they had cataract surgery in the preceding 1 year as this may contribute to postoperative CMO. The betamethasone therapy was stopped if the patients showed increasing intraocular pressure (IOP) >21 mm Hg.
CMO was diagnosed using spectral-domain OCT (SD-OCT) (Spectralis; Heidelberg Engineering, Heidelberg, Germany, and 3D OCT-1000; Topcon, Tokyo, Japan). The criterion of CMO with RP was the presence of visible intraretinal cystoid spaces in the fovea confirmed by horizontal and/or vertical OCT measurement. Spectralis Viewing Module (V.6.0.14.0, Heidelberg Engineering) and OCT viewer (V.2.14, Topcon) were used to measure CFT. CFT was defined as the distance between the vitreoretinal interface and the inner border of RPE, and we regarded it as the average of vertical and horizontal foveal thickness (figure 1). We defined response to treatment as reduction of ≥11% in CFT compared with the right before the start of betamethasone (baseline). Best-corrected visual acuity (BCVA) and IOP were obtained using with full subjective refraction using a Landolt C and a non-contact tonometer for each. When patients visited the outpatient clinic more than one time during the same period, we used the mean values of CFT and BCVA in the same period, because this retrospective study was difficult to match timing. We compared the CFT and BCVA between average baseline and the average of patients meeting inclusion criteria at each period of months 1–3, 5–7, 10–14 and 16–20.
Central foveal thickness (arrow) was defined as the distance between the vitreoretinal interface and the inner border of retinal pigment epithelium at the foveal centre, based on optical coherence tomography imaging at the centre of the fovea.
Statistical analyses
Statistical analysis was conducted using the SPSS statistical software package (V.22). A paired t-test was used to compare the CFT and BCVA average between baseline and 1–3 months period (n=16). A one-way repeated measures analysis of variance with Bonferroni correction for post hoc analysis was used for comparing the CFT and BCVA average of each period (baseline vs 1–3 vs 5–7 months: n=12, baseline vs 1–3 vs 5–7 vs 10–14 vs 16–20 months: n=9). Results were considered significant if P<0.05.
Results
The mean patient age was 39.4±13.8 years (16–51 years; five male and five females). Six patients have CMO in both eyes. Two patients were from families with autosomal dominant form of RP, and eight others were isolated cases wherein the patient was the only affected member of the family. The clinical data were summarised in table 1.
The clinical data of patients with retinitis pigmentosa associated with cystoid macular oedema
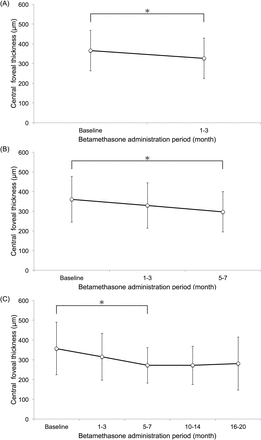
The drugs used as first treatment were brinzolamide or dorzolamide (2 dorzolamide and 14 brinzolamide), during the 3 months prior to betamethasone. Among them, one patient also had received bromfenac at the same time during 2 months in both eyes. Administration periods of betamethasone were 18.9±17.1 months. CMO was mainly found at inner nuclear layer and outer plexiform layer by SD-OCT. CFT was significantly decreased in the 1–3 months period (326±102 µm; n=16; P=0.029; figure 2A) and the 5–7 months period (297±102 µm; n=12; P=0.022; figure 2B and 272±90 µm; n=9; P=0.037; figure 2C) compared with baseline but not in the 10–14 months (271±96 µm; n=9; P=0.485; figure 2C) and 16–20 months periods (281±134 µm; n=9; P=0.289; figure 2C), and the average of CFT data was tabulated in table 2.
Mean of each period central foveal thickness values as measured by optical coherence tomography. (A) The observation period until 1–3 months. A paired t-test result: baseline versus 1–3 months period, P<0.05 (N=16). (B and C) One-way repeated measures analysis of variance with Bonferroni correction. (B) The observation period until 5–7 months: baseline versus 5–7 months period, P<0.05 (N=12). (C) The observation period 16–20 months: baseline versus 5–7 months period, P<0.05 (N=9). *P<0.05. Error bars denote SD.
The average of CFT in each period
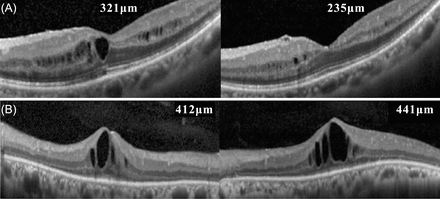
Additionally, we set out the transition of CFT of each patient in online supplementary figure 1. We found effective CFT decreasing in 12 of 16 eyes (81%) as shown in figure 3A, yet ineffective in three eyes among two patients as shown in figure 3B.
Supplementary file 1
The examples of optical coherence tomography (OCT) scanning estimated central foveal thickness (CFT). (A) Patient no. 3: left eye. OCT scanning estimated CFT of a patient who showed an adequate effect of additional betamethasone for 7 months. Left panel is before treatment, and right panel is after treatment. (B) Patient no. 5: right eye. OCT scanning estimated CFT of a patient displaying no effect of additional betamethasone for 7 months. Left panel is before treatment, and right panel is after treatment.
As for the BCVA, there were no significant differences between each group. Logarithm of the minimum angle of resolution (log MAR) (logMAR) BCVA was not significant in 1–3 months period (0.408±0.387 µm; n=16; P=0.535), 5–7 months period (0.447±0.510 µm; n=12; P=1.00), 10–14 months (0.526±0.456 µm; n=9; P=1.000) and 16–20 months period (0.552±0.501 µm; n=9; P=1.000). In three patients, the betamethasone treatment was stopped because of IOP elevation.
Discussion
We showed the therapeutic effects of additional topical steroids for the management of persistent CMO in RP after CAI treatment. The mechanism of CMO in RP is not fully understood and thought to be multifactorial: failure of pumping system in the RPE, a breakdown of the blood–retinal barrier (BRB), intraocular inflammation and vitreomacular traction syndrome (VTS).12 VTS in patients with RP are usually managed surgically; therefore, we excluded such patients from the study.
Many studies have reported the CAI effectiveness, which appears relevant to pumping system improvement. CAI targets membrane carbonic anhydrase intravenous in RPE to acidify subretinal space by accumulation of carbonic acid. It is thought that acidification in subretinal space increases fluid resorption from the retina through the RPE into the choroid.13 Ikeda and colleagues14 studied 16 eyes of nine patients and reported 13 of 16 (81%) eyes showed significant improvement of retinal thickness using topical dorzolamide with positive treatment effects lasting up to 6 months. Fishman and colleagues15 also reported that from 12 patients with CMO in RP, 10 patients (83%) improved both subjective and objective improvement in visual acuity when treated with oral acetazolamide. However, CAI may not adequately demonstrate desired effects or may permit recurrence.9 10 The patients included in our study also did not significantly decrease (≥11%) in CFT using topical CAI. These reports, including our cases, could indicate that CAI alone was not enough in such cases, and other treatment options should be considered.
Another option for managing CMO is with steroids, although it is not fully understood how steroids work for CMO in RP. Numerous groups have reported steroid treatments with several effects including inhibiting immune response that seems relevant to BRB breakdown, reduced levels of inflammatory cytokines and vascular endothelial growth factor. One possibility is that steroids could inhibit some specific antigen–antibody reaction that occurs at the area of failing BRB after the photoreceptor death. Heckenlively and colleagues reported that a breakdown of BRB allowed retinal protein to be released in circulation and sensitised the immune system to attack the retina. Given this observation, steroid therapy may inhibit such immune response16 and result in positive outcomes for treating CMO. We also speculated that steroids would suppress the inflammatory changes in the pathogenesis of CMO in RP.
Furthermore, it was reported in both animal models and human RP subjects that chronic inflammatory reaction may add to the pathogenesis of RP. Yoshida et al 17 found that proinflammatory cytokines and chemokines such as monocyte chemotactic protein-1 (MCP-1), interleukin (IL), C–C motif ligand 5 (CCL5) and tumour necrosis factor-α was elevated in disease modelling RD10 mice. They also reported that various cytokines and chemokines including IL, MCP-1 and CCL were increased in aqueous humour and vitreous fluid by using multiplex ELISA.18 Numerous studies have shown that there was a close relevance between the inflammation and CMO. Intraocular inflammation including cytokines and chemokines reaction disturbed the BRB function and led to CMO.12 Barge and colleagues7 described a case report that triamcinolone via subtenon and intravitreal injection showed temporary benefits in refractory CMO with improvement of visual acuity.Moreover, Lucia and colleagues reported that in non-randomised comparative trail. Intravitreal triamcinolone administration showed decreased central macular thickness.19 Taken together, we speculated that steroids could suppress the inflammatory reaction of CMO in RP. We chose topical use of betamethasone instead of intravitreal or subtenon injection methods because eye-drops are non-invasive and convenient for the patient.
One retrospective study showed recessive genetic disease can predict patient response to CAIs.4 We speculate that steroids may similarly act in accord with genetic inheritance. However, there was no significant relationship among both the autosomal dominant inheritance patients and isolated pattern patients with respect to CFT and logMAR.
In our study, the CFT continued to decrease for the 5–7 months period compared with baseline. The average of CFT from 5–7 months to 16–20 months period was almost unchanged. The lack of significant change between baseline and these periods may be due to small sample size and large SD.
We could not find improvement in visual acuity in this study. Some reports show that visual acuity had improved with the treatment of CMO, while other reports found that visual acuity had not changed. Sandberg and colleagues20 demonstrated that visual acuity was related to parafoveal and foveal retinal thickness in patients with RP. Oishi and colleagues reported there was no correlation between BCVA and total retinal or photoreceptor thickness in 25 RP patients with CMO.21 The reason why there was no relationship between decreasing CFT and BCVA was thought to include the long duration of CMO, which results in photoreceptor damage, and is reflected with suboptimal recovery. However, we believe that lowering CFT holds the promise of preventing some of the risk of reduced visual acuity.
Lenassi et al have reported that there was a strong correlation between retinal sensitivity and outer retinal thickness in patients with RP.22 Ikeda et al reported about macular sensitivity using the automated static perimetry testing (Humphrey Field Analyzer) in CMO with patients with RP. In the condition of reduced retinal thickness from topical dorzolamide, although visual acuity was not significantly improved, macular sensitivity was improved.3 In our study, since additional betamethasone decreased CFT, it is possible that retinal sensitivity improved in these patients.
We found that four eyes in three patients (30%) showed the IOP elevation after using 0.1% betamethasone in this study. The transition of CFT after stopping betamethasone in three eyes of three patients were getting worse (change greater than 10%) for 6 months: in patient no. 3 in the left eye, in patient no. 6 in the right eye and in patient no. 9 in the left eye. We also found CFT stable (did not change more than 10%) in patient no. 6 in the left eye. Numerous studies reported that a rise in IOP may occur as an adverse effect of corticosteroid treatment. IOP is generally thought to rise due to increasing aqueous outflow resistance caused by aggregation of excessive glucocorticoid in trabecular meshwork cells.23 Sapir-Pichhadze et al reported that about one-third of the population had a side effect of IOP elevation with steroid use as a steroid responder.24 While topical betamethasone was effective to treat CMO in RP, IOP may easily elevate and therefore require scrupulous IOP monitoring during the course of betamethasone administration.
We selected SD-OCT to study CMO because previous studies showed a greater sensitivity than FA in detecting CMO. SD-OCT was able to detect CMO in RP, even in eyes with little or no dye leak on FA or minor CMO that is not detectable by ophthalmoscopy.25 Stanga and colleagues26 presented findings showing that OCT imaging was as sensitive as FA for identifying CMO and appropriate method for observing a response to therapy. Additionally, the stress of OCT examination is lower than FA, which is easier for patients.
The present study had several limitations. First, some SD-OCT measurements were only one direction in patients no. 1 (2 of 6 points), no. 7 (1 of 16 point) and no. 8 (1 of 8 point). Second, because the CFT data were collected from different types of SD-OCT measuring instruments, it is possible that the measurement of CFT had minor deviations. Third, because this study was retrospective cohort, we might not follow accurate time course information. Fourth, it is well known that because CMO may have spontaneous remission, it might be possible that the result of decreasing CFT was not only caused by additional betamethasone. Fifth, the eyes of the same individual in six patients may be correlated for the effect of betamethasone. Finally, retrospective cohort studies may result in involving unknown bias that may affect the analysis. Our sample size was relatively small yet informative; therefore, future studies involving more patients are needed to better investigate the appropriate treatment for CMO in patients with RP.
In conclusion, a new possibility for treating CMO with topical betamethasone has been proposed when the primary CAI treatment is not effective. The topical treatment can be safer and more convenient than intravitreal and subtenon injections of triamcinolone. Therefore, we propose additional topical betamethasone to treat persistent CMO in RP.
Acknowledgments
I would like to thank the members of Kobe City Medical Center General Hospital for their hospitality during my visit, where the main results of this paper were obtained.
References
Footnotes
Contributors YH and MT conceived and designed the experiments. SK, YH and ST took an active part in conduct, data analysis and publication drafting the data. SK and ST wrote the manuscript and YH, CK, MF, YK and MT reviewed the manuscript.
Competing interests None declared.
Patient consent Obtained.
Ethics approval This study protocol was approved by the Kobe City Medical Center General Hospital ethics committee.
Provenance and peer review Not commissioned; externally peer reviewed.