Article Text
Abstract
Background To evaluate the efficacy and safety of an extemporaneous preparation of 2% ganciclovir topical eye drops in cytomegalovirus (CMV) anterior uveitis because many studies have confirmed the benefits of topical ganciclovir in varying concentrations.
Design The study employed a retrospective cohort design.
Methods This study enrolled 11 eyes (11 patients) with CMV anterior uveitis. All cases were proved by positive PCR for CMV DNA from aqueous tapping and received topical 2% ganciclovir, applied every 2 hours daily as induction therapy then tapered off and stopped based on clinical response. Outcome measures were best-corrected visual acuity, anterior chamber cell, coin-shaped and other keratic precipitates, intraocular pressure (IOP), the number of antiglaucoma drugs used, the frequency of steroid eye drops used daily and side effects over a 12-month follow-up period. Side effects after applying topical 2% ganciclovir were recorded using questionnaires and eye examination.
Results Mean age was 49.0±17.8 years. IOP, number of antiglaucoma drugs used and keratic precipitates decreased significantly at first week (p<0.013, p<0.024 and p<0.031, respectively) followed by decreased anterior chamber cells and significantly reduced frequency of applying steroid eye drops at 4 weeks (p<0.034 and p<0.017, respectively). Visual acuity significantly improved at 5 months continuously. All clinical improvement was maintained to 12 months, and keratic precipitates were eliminated in 90% of all cases. However, in 27% of discontinued medicine cases, inflammation was recurrent. No significance was observed in all factors between recurrent and non-recurrent groups. The most common side effect was eye irritation (27.27%). No severe complications from the medicine was detected.
Conclusion Extemporaneous preparation topical 2% ganciclovir was effective and safely controlled CMV anterior uveitis. The medication is non-invasive, economical and convenient for hospitals where commercial topical ganciclovir is unavailable.
- cytomegalovirus
- anterior uveitis
- glaucoma
- topical ganciclovir
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
The treatment of cytomegalovirus (CMV) anterior uveitis with topical ganciclovir gained more popularity. The concentration of this medicine was variedly used.
We reported the efficacy of topical 2% ganciclovir in timeline with safety information in these patients.
The findings suggest that the use of extemporaneous preparation topical 2% ganciclovir could be alternatively employed where commercial topical ganciclovir is unavailable.
Introduction
In the past, the known causes of infectious anterior uveitis in the herpesviridae family1 were only varicella zoster and herpes simplex types 1 and 2. Currently, cytomegalovirus (CMV) anterior uveitis and corneal endotheliitis were increasingly found among immunocompetent patients.2 This increase is probably due to advanced modern investigations such as PCR that are able to detect and confirm the diagnosis of infectious uveitis.3 4 CMV anterior uveitis and corneal endotheliitis present anterior chamber inflammation, increased intraocular pressure (IOP), coin shaped lesions or other keratic precipitates (KPs) and iris atrophy.5–7 The diagnosis of CMV anterior uveitis is based on clinical manifestations and PCR results from the aqueous humour.8–11
To treat CMV anterior uveitis, ganciclovir is a well-known medication used to suppress the replication of the herpes virus.12In the beginning, systemic ganciclovir, such as intravenous ganciclovir2 11 and oral valganciclovir,13 14 were recommended for CMV anterior uveitis. However, they are expensive and present the risk of systemic side effects like granulocytopaenia, thrombocytopaenia, anaemia, azoospermia and rising serum creatinine levels.15 Local ganciclovir therapy has become more popular for producing fewer systemic side effects including intravitreal ganciclovir16 and topical ganciclovir. Many studies have confirmed the benefits of topical ganciclovir in varying concentrations from 0.15% ganciclovir17 18 and 0.5% ganciclovir16 19 to 2% ganciclovir.20 21 Commercial topical 0.15% ganciclovir gel is available in many countries; however, in some countries such as Thailand, it has not been imported yet. Therefore, we aimed to evaluate the efficacy of an extemporaneous preparation of 2% ganciclovir eye drops and to determine its safety features as well.
Methods
Medical records in the Ophthalmology Department of Phramongkutklao Hospital were retrospectively reviewed from January 2013 to August 2016. We enrolled 11 patients presented with anterior uveitis, coin-shaped lesions and other KPs, IOP including subjects with a history of using topical steroids and topical antiglaucoma drugs. All patients underwent aqueous tapping using PCR for seven types of herpes, and the results showed only positive for CMV. Patients had a diagnosis of CMV anterior uveitis or corneal endotheliitis based on clinical manifestations and positive PCR results for CMV DNA from the aqueous humour. Patients were treated with topical 2% ganciclovir prepared from 500 mg of Cymevene IV lyophilised powder (Roch, Basel, Switzerland) in 25 mL of sterile water, at 20 mg/1 mL. The price per bottle was 57 GBP. Because this medicine comprises preservative-free eye drops, it expires within 1 month. Furthermore, patients were informed to keep their medication in a brown medicine bottle and in a cool place (2°C–8°C) all the time. Topical 2% ganciclovir was prescribed to be applied every 2 hours daily as induction therapy for 2 weeks and after that tapering off to every 4 hours, four times daily, three times daily, twice a day, once daily every week and finally stopped based on clinical response.
The collected patient data comprised demographics as well as underlying disease and previous ocular treatment including number of topical steroids and antiglaucoma drugs used. Ophthalmic examinations included best-corrected visual acuity (BCVA) using a Snellen eye chart, anterior chamber inflammation, KPs and IOP using Goldman applanation tonometry before and after treatment monthly. BCVA was converted to logMar to record. In addition, anterior chamber cells were graded using SUN classification.22 Antiglaucoma drugs were recorded by counting the number of medications used while steroid use was recorded by counting the frequency of topical 1% prednisolone acetate or loteprednal eye drops in 1 day.
After the patients had used topical 2% ganciclovir for at least 6 months, they completed questionnaires asking about unexpected symptoms including irritation, redness, foreign body sensation, photophobia, dry eye, blurred vision, abnormal discharge and tearing together with questionnaires asking about characteristics of medication change including change in colour or sedimentation. Side effects from using topical eye drops including discharge, conjunctival reaction, punctate epithelial erosion and epithelial defect were also recorded every visit.
The primary outcome was to evaluate the efficacy of topical 2% ganciclovir, while the secondary outcome was to evaluate its safety. All data were recorded using the STATA/MP V.12 Program. Statistical analysis was performed using IBM SPSS statistics, V.23.
This research was approved by the Phramongkutklao Institutional Review Board. Informed consent was obtained from all patients after receiving protocol information.
Results
Clinical responses to topical 2% ganciclovir
Eleven eyes in 11 patients were enrolled in this study. Patient characteristics and demographic data are summarised in tables 1 and 2. The patients comprised eight men and three women, 28–81 years old (49.0±17.8 years). They had the disease before CMV was detected for a mean of 54.0±54.9 months. Two patients underwent penetrating keratoplasty from unknown corneal decompensate; however, they experienced graft rejection and poor visual outcome later. Three of 11 patients (cases 1, 4 and 8) had previously been treated with another route of ganciclovir before quitting because of financial problems.
Characteristics of patients
Characteristics of baseline
Regarding initial clinical presentations (table 3), the average BCVA was 0.2, ranging from 0 to 1.4. Coin-shaped and stellate KPs were detected in 10 eyes (90.9%); mild anterior chamber inflammation was observed in eight eyes (72.7%), while hypopyon was present in one eye (9%) of case 8 receiving oral 2 mg/kg/day of cyclophosphamide therapy. Fundus examinations were normal in all patients. Topical antiglaucoma medications, acetazolamide tablets and 50% glycerine solution were used in 10 patients (90.9%) with an average of three bottles (0.5% timolol, brimonidine and 1% brinzolamide). However, elevated IOPs were still present in six eyes (55%). All cases were using topical steroids, mostly 1% prednisolone acetate except cases 4 and 10 using loteprednal, but at various frequencies, 2–16 times daily to control inflammation.
Clinical manifestations of patients with CMV anterior uveitis included number of antiglaucoma drugs and frequency of steroid eye drops in a day before and after topical 2% ganciclovir therapy
Clinical response to topical 2% ganciclovir therapy at 12 months compared with clinical manifestations before starting medication is shown in table 3. All cases showed positive responses to therapy either improved BCVA, decreased anterior chamber cells from 0.5 to 0, eliminated KPs, decreased IOP from 28 to 12 mm Hg, reduced number of antiglaucoma drugs from 3 to 0 bottles and reduced frequency using steroid eye drops from 4 to 0 times daily (tables 4 and 5). Two cases (cases 5 and 9) underwent trabeculectomy with mitomycin C because of uncontrolled IOP and visual field defect progression (18.18%). One case (case 5) discontinued topical 2% ganciclovir at 6 months after the patient decided to choose commercially available 0.15% ganciclovir gel after filtering surgery. One case was lost to follow-up after 6-month visit. At 12 months, 4 of 11 eyes (36%) could discontinue topical 2% ganciclovir, while 5 of 11 eyes (45%) were treated three to four times daily for maintenance therapy. In addition, 6 of 11 eyes (0.55%) used antiglaucoma drugs (0.5% timolol and 1% brinzolamide) long term as well.
Clinical response of 2% ganciclovir eye drop compared with baseline 1 week–6 months
Response of 2% ganciclovir eye drop compared with baseline 7 months–12 months
After administering topical 2% ganciclovir, IOP and KPs were rapidly controlled in 1 week with significance (p<0.013 and p<0.031) compared with baseline leading to a decrease in antiglaucoma medication and thereafter significantly (p<0.024) as well (table 4). Anterior chamber cells were significantly controlled at 4 weeks (p<0.034) simultaneously as the frequency of steroid eye drops was significantly reduced (p<0.017). BCVA was the last to improve significantly at 5 months (p<0.020).
At 4 weeks of administering topical 2% ganciclovir, IOP and anterior chamber inflammation were significantly controlled compared with baseline every month regularly. The details of each case are shown in figures 1–11.
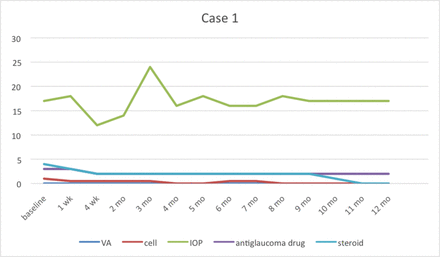
Case 1 had recurrent symptoms at the 3rd month then we stopped using topical 2% ganciclovir at 11th month. IOP, intraocular pressure; VA, visual acuity.
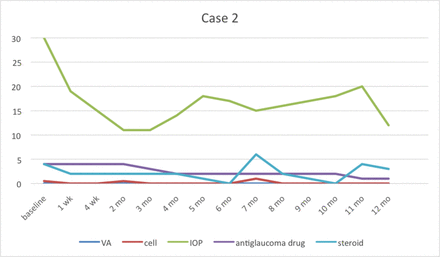
Case 2 had recurrent symptoms at the 7th and 11th months while tapering 2% topical ganciclovir. IOP, intraocular pressure; VA, visual acuity.
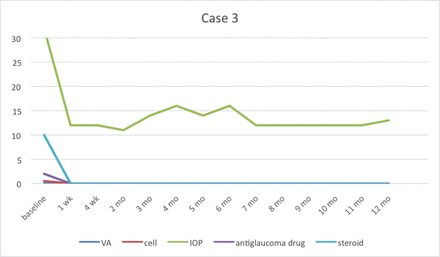
Case 3 could be stopped using 2% topical ganciclovir at 3rd month without any recurrent symptoms. IOP, intraocular pressure; VA, visual acuity.
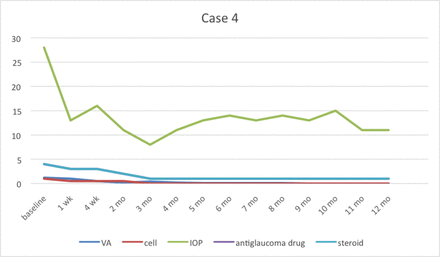
Case 4 (corneal decompensate) still continues topical ganciclovir four times a day for undergoing penetrating keratoplasty in the future. IOP, intraocular pressure; VA, visual acuity.
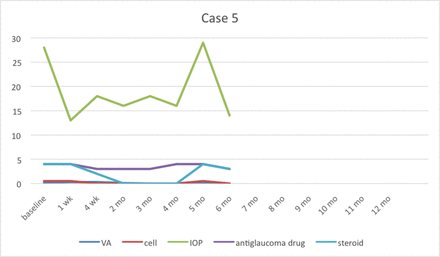
Case 5 had recurrent symptoms at fifth month and then finally the patient stopped taking medicine. IOP, intraocular pressure; VA, visual acuity.
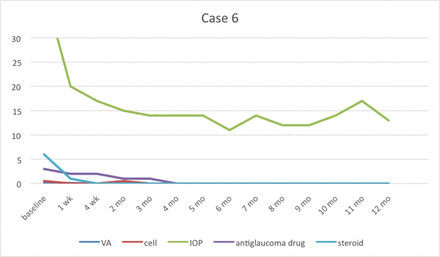
Case 6 stopped taking 2% topical ganciclovir at 7th month. After that the recurrent symptoms occurred at 10th month by the presenting of keratic precipitates. IOP, intraocular pressure; VA, visual acuity.
Case 7 stopped taking topical 2% ganciclovir at 6th month then recurrent symptoms occurred at 9th month. IOP, intraocular pressure. VA, visual acuity.
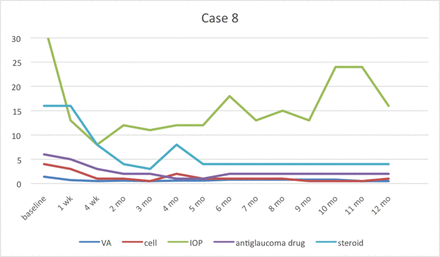
Case 8 had no recurrence; however he was still on medicine. At 10th month follow-up, the patient had poor compliance to take antiglaucoma medicine. IOP, intraocular pressure; VA, visual acuity.
Case 9 could be stopped using 2% topical ganciclovir at 11th month without any recurrent symptoms. IOP, intraocular pressure; VA, visual acuity.
Case 10 could be stopped using 2% topical ganciclovir at 11th month without any recurrent symptoms. IOP, intraocular pressure; VA, visual acuity.
Case 11 stopped taking medicine by herself at 2 weeks then the recurrent symptoms occurred at 5th month. After that topical 2% ganciclovir was restarted. IOP, intraocular pressure; VA, visual acuity.
Six of 11 cases were recurrent: three cases were recurrent when they stopped using topical steroid eye drops but were still using topical 2% ganciclovir three times daily,while three cases were recurrent after stopping topical 2% ganciclovir (27%). They tended to present poorer VA, less anterior chamber cells and more KPs in the recurrent group; however, no significance was observed in all factors between the recurrent and non-recurrent groups (table 6).
Factors between recurrent and non-recurrent group
Side effects of topical 2% ganciclovir treatment (tables 7 and 8)
The most common complaint of symptoms was irritated eye (27.27%), lasting 1–2 min after applying eye drops. The second symptom was tearing (18.18%). Other symptoms including red eye, foreign body sensation, eyelid swelling and temporary blurred vision were also recorded (9.09%). No systemic or serious complications were observed in any of the patients.
Clinical manifestations after using topical 2% ganciclovir treatment
Ocular examination after using topical 2% ganciclovir treatment
Regarding medication (table 9), 2 of 11 patients (18.18%) complained about a small amount of white sediment appearing even though the medication was stored in a cool place. It returned to clear after shaking. Any colour change in medication was undetected.
Characteristic of medication change
Discussion
Ganciclovir is a synthetic purine nucleoside, an analogue of quanosine23 and a well-known medication against CMV. Topical forms of ganciclovir have shown effective penetration through the cornea including the aqueous humour24 25 and comprise the first treatment option in CMV anterior uveitis and CMV endotheliitis.
Most studies have demonstrated the efficacy of systemic and topical ganciclovir, but not in timeline details. In particular, ours was the first study to evaluate the response of topical ganciclovir in the first week and then monthly. Although our study showed reduced IOP was the first response when using topical 2% ganciclovir, 18.18% of cases required a trabeculectomy with mitomycin C. In addition, Wong et al reported 21.2% of cases developed uncontrolled IOP and underwent glaucoma surgery.
Some studies18 19 have specified that KPs and anterior chamber inflammation improved after a 4-week administration of topical ganciclovir similar to our study. Tapering off topical steroid eye drops needs to be slow along with anterior chamber inflammation. That is why at 12th month, some patients were still using once to twice daily. However, BCVA did not improve significantly at 12 weeks, while our study showed it improved significantly at 5 months. Hence, even though other anterior chamber inflammation was controlled, BCVA was the last significant continuous response.
Chee and Jap found that topical ganciclovir had lower recurrence rates than systemic ganciclovir.26 In this study, we found 27% of discontinued medication was recurrent in anterior uveitis, similar to Koizumi et al reporting 36% of cases showed recurrence of inflammation and anti-CMV treatment was repeated14 as well. Wong et al reported topical ganciclovir reduced the number of episodes of recurrent anterior uveitis17 while using and suggested that maintenance therapy may be required to prevent recurrences27 because ganciclovir, a virustatic agent, does not eradicate viruses in the latent phase.
Our study found unexpected symptoms after using topical ganciclovir; however, they were insignificant and improved by adding artificial tears. No studies reported significant side effects such as ocular discomfort or corneal toxicity from topical ganciclovir 0.15%,17 18 0.514 or 2%20 as well. White sediment appeared in some bottles. We recommend shaking the medicine while mixing very well because ganciclovir has relatively small-sized molecules with high lipophilicity causing ganciclovir to be poorly soluble in water.25
Limitations in this study included being a relatively small case series and retrospective non-randomised study. Furthermore, the study should compare the efficacy and safety of other concentrations of topical ganciclovir in a randomised control trial requiring longer term follow-up.
Conclusion
Topical 2% ganciclovir alone is a safe and effective medicine to control inflammation in CMV anterior uveitis. The first response is reduced IOP and KPs, and then decreased anterior inflammation. Therefore, extemporaneous preparations of topical 2% ganciclovir are non-invasive, economical and convenient for the hospital where commercial topical ganciclovir is unavailable.
References
Footnotes
Competing interests None declared.
Patient consent Obtained.
Ethics approval The Phramongkutklao Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.