- Raffaela Mistò1,
- Laura Giurgola2,
- Francesca Pateri1,
- Elisabetta Frigerio1,
- Anna Limongelli1,
- Jana D’Amato Tóthová2
- 1Eye Bank of Monza, San Gerardo Hospital, Monza (MB), Lombardia, Italy
- 2Research and Development, Alchilife S.r.l., Ponte San Nicolò (PD), Italy
- Correspondence to Dr Jana D’Amato Tóthová; JTothova{at}alchilife.com
- Received 31 May 2017
- Revised 5 October 2017
- Accepted 10 October 2017
Abstract
Objective This study aimed at validating the method for sterility testing of the corneal culture medium, TISSUE-C, and the transport/deswelling medium, CARRY-C, according to the method suitability test, as defined by the European Pharmacopoeia (EP), using RESEP, which is a new medical device for removal of antimicrobial agents and an automated culture system.
Methods and analysis The six EP reference strains were inoculated in TISSUE-C and CARRY-C. Half of the samples were treated with RESEP (RESEP+ group) prior to the sterility testing, whereas the remaining samples were untreated (RESEP− group). Growth controls were obtained by direct inoculation of the micro-organisms in the culture broths. Microbial growth was read by an automated light scattering culture system within 48 hours.
Results The use of RESEP allowed detection of microbial growth in 100% of the tested samples, with a mean time to detection (TTD) comparable with that of the growth control group. Significantly lower sensitivity (38.83%±20.03% for both media, P<0.05) and TTD variability, depending on the tested micro-organism, were observed in the RESEP− group. The method specificity was 100% for both groups.
Conclusion The use of RESEP increased the sensitivity of the sterility testing method to 100% and, for the first time, allowed validation of the method for sterility testing of corneal storage media according to the EP method suitability test. This further increases the safety of the corneas intended for transplantation.
- cornea
- diagnostic tests/investigation
- microbiology
- infection
- eye (tissue) banking
- ocular surface
Key messages
What is already known about this subject?
The antimicrobials in corneal storage media may cause false negative findings during sterility testing and compromise the safety of corneas intended for transplantation.
What are the new findings?
The use of the RESEP device for removal of antimicrobials from samples allowed validation of the method for sterility testing of the corneal storage media according to the method suitability test described by the European Pharmacopoeia (EP).
How might these results change the focus of research or clinical practice?
A validated microbiological method according to the EP may be easily transferred to other automated instruments and media for routine sterility testing in order to avoid false negatives and to further increase the safety of the corneas intended for transplantation.
Introduction
The cornea is the most commonly transplanted tissue worldwide. Progress in the surgical techniques such as the use of lamellar grafts has led to a significant improvement in graft survival and patient outcome.1–3 However, corneal transplantation is still associated with, although low, a risk for endophthalmitis,4 which can lead to reduced graft survival and poor visual outcomes.5 Because endophthalmitis may result from contaminated corneal tissue transplants,6 each bank selects the most meticulous processes for procurement, processing, storage and transport of corneal tissues in order to minimise the risk of disease transmission from donors to allograft recipients.2 3 6 Even if the procedures vary from bank to bank, proper management of the risks must include the use of validated microbiological methods with accurate sensitivity and specificity to detect the pathogenic micro-organisms in the corneal storage and transport media, which may affect the safety of the tissue to be transplanted.2 6
Most of the European eye banks store corneal tissues in an organ culture medium at 31°C to allow survival of the corneal endothelial cells for up to 4 weeks.2 7 8 The presence of antimicrobial agents in the corneal storage and transport media reduces the risk of micro-organism proliferation.6 However, the antimicrobial agents may induce bacteriostasis in the samples that undergo sterility tests and lead to false negative (FN) results during microbiological analysis. Both the European Pharmacopoeia (EP)9 and the United States Pharmacopoeia10 recommended elimination of any factor that may interfere with microbial growth during sterility testing of the samples. Because no specific standards for sterility testing are available in tissue banking, each tissue bank validates its own method.11–13
In the present validation study of the method for sterility testing of the corneal culture media, we used an automated system with light scattering technology that allowed rapid detection of microbial growth. The system was originally designed for bacterial screening of urine and other biological samples and was recently reported to be useful for the analysis of fluid samples,14 including corneal storage media.15 However, the system does not eliminate or neutralise the antimicrobial residues present in the corneal storage media. Therefore, in this validation study, we included an additional treatment of the sample using the RESEP device in order to remove the factors that could interfere with microbial growth in the samples, as recommended by EP, before running the test in the automated system.
Materials and methods
Experimental design
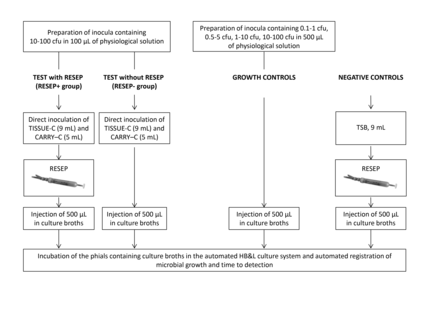
The experimental design is summarised in figure 1.
Experimental design of the study. Samples of TISSUE-C (9 mL) and CARRY-C (5 mL) previously spiked with 10–100 colony-forming units (cfu) of known micro-organisms were either treated with RESEP or left untreated; then, 500 µL samples of TISSUE-C and CARRY-C media (containing known micro-organisms at 0.5–5.5 and 1–10 cfu, respectively) were injected into the HB&L KIT phials for automated reading. Inocula containing different amounts of microbial strains, which were used as growth controls, were injected directly in the HB&L KIT phials; 500 µL samples of sterile TSB (treated with RESEP) were used as negative controls.
Media, instruments and devices
TISSUE-C and CARRY-C (AL.CHI.MI.A., Ponte San Nicolò, Italy) are commercially available CE-marked media that are intended for corneal storage at 31°C and cornea transport/deswelling at room temperature, respectively. Both media contain penicillin G, streptomycin sulfate and amphotericin B. In addition, CARRY-C contains dextran as a deswelling agent.
RESEP (AL.CHI.MI.A.) is a syringe-like, patented, CE-marked device, which contains a resin mixture for removal of antimicrobial agents from liquid samples. It was specifically designed to avoid FN results in the microbiological tests performed by the tissue banks and had been validated by the manufacturer for total elimination of antimicrobials from the CARRY-C and TISSUE-C media. The device was used according to the manufacturer’s instructions. Briefly, the sample was withdrawn using the RESEP syringe and then incubated at room temperature for 20 min under stirring; thereafter, the sample was inoculated in the HB&L KIT (Alifax, Polverara, Italy) phials that contained culture broths. The culture broths that were used specifically with the automated HB&L system (Alifax) for detection of micro-organisms included the HB&L CULTURE KIT for common aerobic bacteria, the HB&L SABOURAUD KIT for aerobic fungi and the HB&L ANAEROBE KIT for obligate or facultative anaerobic bacteria. The automated HB&L system is a commercially available light-scattering technology for automated bacterial culture of biological fluids; it automatically records the time to detection (TTD) of microbial growth.
Preparation of the inocula
The following EP reference strains were acquired from the American Type Culture Collection (ATCC, Manassas, VA USA): Clostridium sporogenes (ATCC 19404), Staphylococcus aureus (ATCC 6538), Candida albicans (ATCC 10231), Pseudomonas aeruginosa (ATCC 9027), Aspergillus brasiliensis (ATCC 16404) and Bacillus subtilis (ATCC 6633). An inoculum solution containing 10–100 colony forming units (cfu) of each microbial strain in 100 µL of sterile physiological solution (0.9% (m/v) NaCl in H2O) was prepared to inoculate the medium samples, whereas 1–10, 0.5–5 and 0.1–1 cfu in 500 µL of sterile physiological solution was prepared for inoculation of the culture broths as growth controls; all inocula were prepared from lyophilised pellets under sterile conditions according to the manufacturer’s instructions.
All inocula were also plated on Tryptone Soy Agar or Sabouraud Chloramphenicol Agar (Biogenetics, Ponte San Nicolò, Italy) (n=5) and incubated at 37°C and 25°C, respectively, for 24–48 hours. The number of cfu was counted, and the actual inoculum concentration was determined for each micro-organism.
Preparation of samples for sterility tests
Sample preparation was performed under sterile conditions. For each micro-organism, 100 µL of the inoculum solution containing 10–100 cfu were injected into six phials containing 9 mL of TISSUE-C and six phials containing 5 mL of CARRY-C. The volume of the samples represented 9.0% and 10.0% of the total volume of TISSUE-C and CARRY-C, respectively.
Sterility test using RESEP (RESEP+ group)
For each microbial strain, three replicates of TISSUE-C and CARRY-C samples were treated with RESEP under continuous stirring at room temperature for 20 min. Subsequently, 500 µL of each sample was injected in the HB&L KIT phials; the HB&L SABOURAUD KIT was used for A. brasiliensis and C. albicans, the HB&L ANAEROBE KIT was used for C. sporogenes and the HB&L CULTURE KIT was used for S. aureus, P. aeruginosa and B. subtilis. According to the manufacturer’s instructions, the maximum sample volume was 500 µL. The inoculated HB&L KIT phials were then incubated in the automated HB&L system at 37°C until a positive reading was obtained or until 48 hours, in accordance with the manufacturer’s instructions. The incubation time in the HB&L system was extended to 5 days for A. brasiliensis only.
Sterility test without RESEP (RESEP− group)
For each microbial strain, 9 mL of TISSUE-C and 5 mL of CARRY-C samples were inoculated in triplicate. RESEP treatment of the media was skipped, and 500 µL was withdrawn, injected directly in the corresponding HB&L KIT phials and incubated in the automated HB&L system.
Negative controls
For negative controls, 9 mL of sterile Tryptone Soy Broth (Biogenetics) was treated with RESEP under continuous stirring at room temperature for 20 min; 500 µL of the medium was injected directly in the corresponding HB&LKIT phials and incubated in the automated HB&L system. For each HB&LKIT, nine replicates (phials) were assessed in four different experiments.
Growth controls
As growth controls for each microbial strain, the inocula containing 10–100, 1–10, 0.5–5 and 0.1–1 cfu in 500 µL of sterile physiological solution were injected directly, at least in triplicate, in the HB&LKIT phials containing optimal growth broths.
Data analysis and statistics
The percentage of positive readings by the HB&L system was calculated for each group, and each microbial strain was tested. Fisher’s exact test was used to compare the number of positive samples between the groups using 2×2 contingency tables. The mean TTD and SEs were calculated for each of the tested conditions and were presented in box plots.
The difference in TTD between groups were analysed for each micro-organism using the Kruskal-Wallis one-way analysis of variance by ranks. The Dunn’s post hoc test was performed for non-parametric, pairwise, multiple comparisons in the independent groups. A P value of <0.05 was considered statistically significant.
The mean sensitivity of the method for RESEP+ and RESEP− groups was expressed as a percentage and was calculated as true positive (TP) results divided by the TP plus FN result ([TP]/[TP + FN]). The specificity was expressed as a percentage and was calculated as the true negative (TN) results divided by the TN plus false positive (FP) results ([TN]/[TN + FP]).
Results
Detection of microbial growth
Table 1 shows the percentage of the TISSUE-C and CARRY-C positive samples for each microbial strain in the RESEP+ and RESEP− groups and in comparison with the growth controls. In the RESEP+ group, all samples (100%) in both media were positive for all the tested micro-organisms. Similarly, the growth controls containing the same or higher amount of cfu than those contained in the tested samples (0.5–5, 1–10 and 10–100 cfu) yielded 100% detection of microbial growth. The RESEP+ samples were not significantly different from their respective growth controls (P>0.05).
Percentage of positive samples detected by the HB&L system (sensitivity) for all tested conditions
In the RESEP− group, 100% microbial growth of only C. albicans and A. brasiliensis was detected in the TISSUE-C and CARRY-C samples. None of the samples inoculated with B. subtilis, P. aeruginosa and S. aureus in the RESEP− group showed microbial growth (P<0.05), whereas C. sporogenes showed microbial growth only in 33% of the tested TISSUE-C and CARRY-C samples. All the negative control samples showed absence of microbial growth.
In the growth controls containing 0.5–5, 1–10 and 10–100 cfu, 100% microbial growth of all the tested strains was detected. At a concentration of 0.1–1.0 cfu, the maximum detection corresponded to 67% for C. albicans.
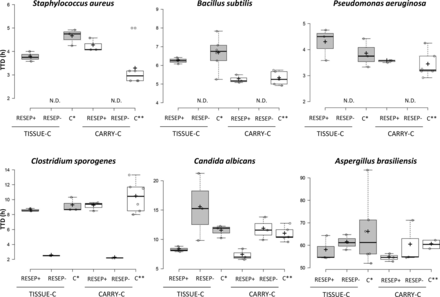
Time to detection
Figure 2 shows the box plot of the TTD for each tested micro-organism in both TISSUE-C and CARRY-C media, compared with the growth controls. In the RESEP+ group, the TTD ranged from 3.56±0.03 to 9.17±0.34 hours for both media, and the mean TTD was comparable with that of the growth controls for S. aureus, B. subtilis, P. aeruginosa and C. sporogenes (P>0.05). In the RESEP− group, S. aureus, B. subtilis and P. aeruginosa were not detected by the automated system in the TISSUE-C and CARRY-C samples. The TTD was comparable with that of the growth controls for C. albicans and A. brasiliensis only (P>0.05). However, after 48 hours, there was no growth of A. brasiliensis in all of the samples, including the growth controls; for this reason, incubation time was extended to 5 days in order to obtain a positive reading and TTD value.
Box plots for the time to detection. Values were calculated for each tested micro-organism in both TISSUE-C and CARRY-C media and were compared with those of the growth controls. +, mean value; C*, growth control 0.5–5 cfu; C**, growth control 1–10 cfu; N.D., not detected; TTD, time to detection.
Sensitivity and specificity of the method
As shown in table 2, the mean sensitivity was 100%±0.00% for both the tested media in the RESEP+ group. The RESEP− group showed a significantly lower sensitivity (38.83%±20.03% for both TISSUE-C and CARRY-C, P<0.05) and great variability that ranged from 0% to 100%, depending on the tested micro-organism (table 1). The specificity was 100%±0.00% for both groups.
Method sensitivity and specificity obtained by the HB&L System for TISSUE-C and CARRY-C media in the RESEP+ and RESEP− groups
The sensitivity of the automated system was assessed at different levels of contamination without any interfering factors (growth controls). For all the tested microbial strains, the sensitivity was 27.9% at 0.1–1 cfu contamination level and 100% at higher contamination levels (0.5–5, 1–10 and 10–100 cfu).
Discussion
The automated HB&L system for microbiological analysis of biological samples may provide an important advantage to tissue banks that perform sterility testing of samples that are free from antimicrobials, because it allows rapid analysis with high method sensitivity and high sample throughput.15–17 However, the system showed relevant limitations for the analysis of corneal storage media that contained antimicrobials. First, unlike other automated systems such as the BACTEC blood culture system,18 the HB&L system does not implement any antimicrobial removal or neutralisation process; second, as reported by other authors, the sample volume is restricted to 500 µL.15 Our findings showed that the presence of streptomycin sulfate and penicillin G, which are known to be effective in reducing the bioburden of contaminated tissues,19 in the tested media clearly interfered with the microbial growth during sterility testing and resulted in FN results. In fact, the automated system did not detect the presence of four (B. subtilis, C. sporogens, P. aeruginosa and S. aureus) out of six microbial strains after inoculation in TISSUE-C and CARRY-C media at 1–10 and 2–20 cfu/mL, respectively.
Because both the tested media contained the same antimicrobials, all the sterility test results were comparable between TISSUE-C and CARRY-C, as expected. Conversely, the same samples showed 100% growth of all the strains tested after removal of the antimicrobials with RESEP; without removal of the antimicrobials, only the samples contaminated with C. albicans and A. brasiliensis were found positive. The unchanged fungal growth may be explained by the low concentration of the antifungal agent in the media or by a reduced sensitivity of these micro-organisms to the antimicrobials.
In the absence of antimicrobials (growth controls), the detection limit of the automated system was determined as greater than or equal to 1–10 cfu/mL; the same detection limit was obtained for both tested media only after RESEP treatment for antimicrobial elimination. The results with a lower contamination level of 0.2–2 cfu/mL were under the system detection limits because only 27.9% of the samples were detected positive in the absence of antimicrobials.
The HB&L KIT phials used with the automated system allowed very restricted sample volumes of 500 µL that could result in a non-representative sampling of the tested media; therefore, the sampling procedure should also be validated in the eye banking practice.
Excluding the FN results for which the TTD could not be obtained, the TTD was relatively fast because microbial growth was detected in a matter of hours and showed minimal variability within groups for all micro-organisms, except for A. brasiliensis.
The difficulty of proving the presence of fungal contamination in culture media has been a matter of controversy.9 We showed that detection of A. brasiliensis with the automated system required more than 48 hours of incubation in the growth control samples. Delayed detection of A. brasiliensis, compared with that of other micro-organisms, was probably due to the slower mycelial growth pattern, which differs significantly from bacterial growth.20 21 Surprisingly, even if A. brasiliensis growth was grossly visible within 48 hours, the results remained undetected by the system. Limitations of the automated system in detecting filamentous fungi have also been observed by other authors.15 In our study, the system showed positive readings for A. brasiliensis when the incubation time was extended to 5 days. Therefore, according to our findings, the incubation time of the sample should be extended from 48 hours to 5 days at least, in order to include the detection of filamentous fungi.
Similarly to Buzzi et al,22 we wanted to verify whether the microbiological positivity observed in the RESEP+ group could have been a consequence of some additional manipulation. However, we excluded this possibility because none of our negative control samples that underwent identical manipulations was found positive for microbial contamination. Moreover, according to our data, RESEP does not remove micro-organisms from the samples even at low contamination levels. In fact, all the samples contaminated with 1–10 or 2–20 cfu/mL, and subsequently treated with RESEP, showed microbial growth after inoculation of the growth broths.
Other studies attempted to validate the method for the sterility testing of the corneal culture media using automated BACTEC blood culture system7 8 18 and showed important method limitations in microbial growth due to the high content of residual antimicrobials.
In agreement with the previous studies,7 8 18 22 we showed that the presence of antimicrobial agents in the corneal storage and transport media may significantly reduce the sensitivity of sterility testing in liquid samples and may lead to FNs. The use of RESEP for the removal of residual antimicrobials from liquid samples increased the sensitivity of the sterility testing method to 100% for all six EP reference microbial strains. To the best of our knowledge, the use of RESEP device therefore allowed, for the first time, validation of a method for sterility testing of the corneal storage and transport/deswelling media according to the method suitability test of the EP. The validated method in the present study refers to sterility testing of 9 mL of the corneal storage medium, TISSUE-C, and 5 mL of the deswelling/transport medium, CARRY-C, using the RESEP device, prior to the automated analysis with the HB&L system with 5 days incubation period. Sterility testing could not be validated in compliance with the same standards without the use of RESEP. The same validation approach could be easily reproduced and routinely applied to other automated instruments and human tissue and cell storage media containing antimicrobials, thereby increasing the method sensitivity and the safety of the tissues and cells intended for transplantation.
Acknowledgments
We would like to thank Cristina Gianotti for the writing assistance and Sabrina Molena for the excellent technical assistance.
Footnotes
Contributors RM and JDT planned and designed the study, analysed the data, drafted the manuscript and critically revised the manuscript. LG, FP, AL and EF acquired, analysed and interpreted the data, as well as drafted and critically revised the manuscript. JDT submitted the manuscript.
Competing interests JDT and LG are employed by the company involved in the research and development of one medical device discussed in the present paper. RM, FP, AL and EF have no financial or proprietary interest in any of the materials or methods mentioned.
Patient consent Patient consent was not required because this study did not involve human subjects.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.