Article Text
Abstract
Objective This study aimed to determine whether treatment with the 0.2 µg/day fluocinolone acetone implant (FAc; ILUVIEN, Alimera Sciences) and the associated improvements in best-corrected visual acuity (BCVA) and central subfield thickness (CST) demonstrated in the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study have an impact on the patient’s decision to drive as measured by the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25).
Methods This was a post hoc analysis of up to 3 years of NEI-VFQ-25 data collected during the phase III FAME trial. Patients were divided into four quartiles according to baseline NEI-VFQ-25 driving subscale (DSS) score. Patients who had never driven were excluded. Patients received either the 0.2 µg/day FAc implant or sham (dummy injection). Change in the DSS score of the NEI-VFQ-25 questionnaire over 3 years in FAc-treated versus sham-treated patients was analysed by BCVA, CST and baseline DSS score.
Results The proportion of patients achieving BCVA≥20/40 was similar between the FAc and sham groups throughout the study, while improvements in CST were significantly greater in the quartile of FAc-treated patients with the lowest baseline DSS score (quartile 1; p=0.04). Significant improvements in DSS score were also observed in quartile 1 (p=0.024), while numerical—but not significant—improvements in DSS score were observed in the full cohort.
Conclusion This post hoc analysis demonstrates a significant association between clinical outcomes in diabetic macular oedema and improvement in quality of life measures following a single FAc implant.
- Retina
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
It is known that decreased vision due to diabetic macular oedema (DMO) can have a substantial effect on quality of life (QoL) as measured by the driving subscale score of the NEI-VFQ-25 National Eye Institute Visual Functioning Questionnaire-25 questionnaire.
There is potential for improvement in vision-related QoL metrics following treatment of DMO.
What are the new findings?
In this analysis, we demonstrate a significant association between better visual acuity and oedema control in DMO and patient’s decision to drive over a period of 3 years following treatment with a single sustained release fluocinolone acetone implant compared with sham.
How might these results change the focus of research or clinical practice?
This illustrates the potential for significant and sustained improvement in quality of life with treatment of DMO.
Introduction
Poor or declining vision can have a substantial effect on quality of life (QoL), as has been demonstrated in visual diseases such as glaucoma,1 Leber’s hereditary optic neuropathy, age-related macular degeneration and diabetic macular oedema (DMO).2 3 Effects can be wide ranging and span interpersonal relationships, career prospects and psychological well-being. Consequently, demonstrating the efficacy of ophthalmic therapies on QoL measures is an important consideration.4 5
The 0.2 µg/day fluocinolone acetonide (FAc; ILUVIEN, Alimera Sciences) implant is currently the only approved long-term, sustained-release, injectable drug delivery system for treatment of DMO.6 The implant provides 3 years of continuous FAc microdosing,7–9 which provides the potential to control oedema consistently over several years following a single injection.
The Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study (NCT00344968) demonstrated the efficacy of 0.2 µg/day FAc implant compared with sham injection in subjects with DMO.10 At year 3, 28.7% of patients in the 0.2 µg/day FAc group had an improved best-corrected visual acuity (BCVA) of ≥15 early treatment diabetic retinopathy study (ETDRS) letters compared with 18.9% of sham-controlled patients (p=0.018).11
Driving cessation may have a considerable effect on patient welfare.12 Various studies have noted the negative consequences of driving cessation on health-related QoL, mental health and the ability to access healthcare resources and attend medical appointments.13–15 In this way, driving cessation due to DMO may have a negative impact on the management of diabetes and its comorbidities. Consequently, the achievement of driving vision (in all but three US states the minimum driving vision for the provision of an unrestricted driving license is 20/40 Snellen16; however, the driving vision considered for restricted licenses varies from state to state17) is of potential clinical importance and is often cited as an indicator of the clinical effectiveness in DMO treatments.18–20 However, the achievement of legal driving vision is not the only factor in the patient’s decision to drive. Vision-related QoL factors, such as low confidence, may negatively affect the decision to drive in individuals with legal driving vision.21
The National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) is a validated health-related questionnaire that contains subscales related to different QoL measures.22–25 The questionnaire consists of 25 questions, which are grouped into subscales that capture the impact of vision on different aspects of the patient’s life, including the decision to drive and perception of driving difficulties. Higher NEI-VFQ-25 scores indicate better vision-related QoL, and published literature in patients with DMO has demonstrated the sensitivity of NEI-VFQ-25 score improvements to visual acuity gains.5
In this exploratory post hoc analysis of the results from the FAME study, associations between changes in NEI-VFQ-25 driving subscale (DSS) scores and clinical outcomes were investigated in order to determine the real-world impact of the 0.2 µg/day FAc implant.
Materials and methods
The FAME study consisted of two parallel, multicentre, clinical trials conducted in the USA, Canada, India and Europe. Details of the study design and patient population have been reported previously.10 Patients or the public were not involved in the study design, conduct or reporting.
Patients received either 0.2 µg/day FAc or sham. The sham injection was delivered by pressing the hub of the delivery device against the sclera with a similar pressure to that used for the delivery of the implant. Both the 0.2 µg/day FAc group and the sham group were permitted laser treatment at 6 weeks if there was no improvement in oedema. Additional laser treatments could be administered at later visits on the judgement of the investigator.
For this exploratory post hoc analysis, FAME patients who met the following criteria were included: (1) BCVA assessed bilaterally at baseline (equal or better baseline BCVA in the study eye compared with the fellow eye; vision-related patient-reported outcomes are often affected by the better-seeing eye,26 particularly in binocular activities such as driving) and (2) NEI-VFQ-25 scores at either year 2 or year 3 with no missing answers for questions 15 or 16 (DSS). Investigators read out the questionnaire to vision-impaired patients. Patients were excluded from the analysis if they had never driven, as assessed by question 15a of the NEI-VFQ-25.
Demographics and baseline characteristics were summarised for all subjects based on their baseline NEI-VFQ-25 DSS score. Changes in NEI-VFQ-25 DSS score were summarised for all subjects who had a baseline and at least one post-baseline assessment.
Possible ceiling effects caused by the inclusion of data from patients who may have limited scope for improvement due to high NEI-VFQ-25 scores at baseline were investigated by dividing the cohort into quartiles according to baseline DSS scores: quartile 1 (scores 0–25; greatest difficulty driving); quartile 2 (scores>25–50); quartile 3 (scores>50–75); quartile 4 (scores>75–100; least difficulty driving). Changes in NEI-VFQ-25 DSS outcomes were compared between patients receiving 0.2 µg/day FAc or sham injection for the four quartiles.
Patients completed the NEI-VFQ-25 questionnaire, including the DSS questions (online supplementary table 1), at baseline, year 2 and year 3. Changes over time in DSS score up to 3 years were reported. Area under the curve (AUC) was calculated for central subfield thickness (CST), which was assessed using time domain optical coherence tomography (OCT; Stratus OCT, Carl Zeiss Meditec, Dublin, California, USA). Outcomes for the other NEI-VFQ-25 subscales of general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, colour vision and peripheral vision were also evaluated.
Supplemental material
Categorical variables were described using frequencies and percentages, and quantitative variables using descriptive statistics (mean and either SD or SE).
Differences between mean changes from baseline in NEI-VFQ-25 subscale scores were analysed using an analysis of variance model with treatment group as the fixed effect.
Results
Data for 70 patients from the FAME study who met the inclusion criteria were included in this post hoc analysis; 45 patients were treated with 0.2 µg/day FAc and 25 patients were treated with sham injection. This cohort was divided into quartiles according to baseline DSS scores. Baseline demographics and disease characteristics are presented in online supplementary table 2.
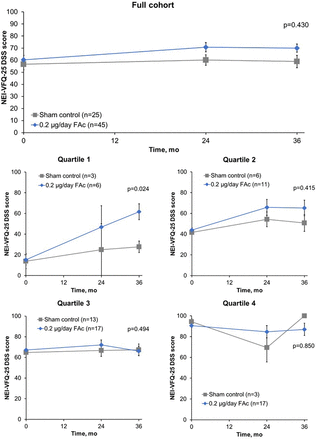
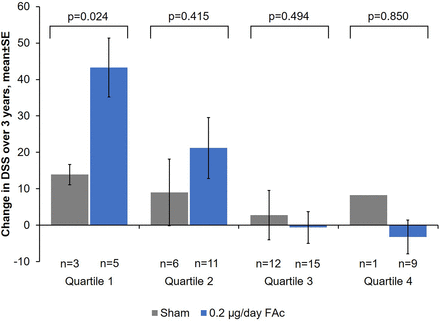
For the full cohort, DSS scores showed numerical improvement over time for those patients receiving 0.2 µg/day FAc implant versus sham control (figure 1). The change in DSS score was assessed by mean baseline DSS quartiles to control for any possible ceiling effects; DSS scores for each of the quartiles revealed that all FAc-treated patients experienced some improvements in DSS scores over time compared with sham-treated patients (figure 1). These improvements over sham treatment at year 3 only reached significance for patients receiving FAc in quartile 1, with a mean (95% CI) difference of −31.1 (−56.3 to −5.8; p=0.024; figure 2). A non-significant, numerical trend in favour of FAc was observed for quartile 2 patients with an observed mean (95% CI) difference of −11.1 (−38.9 to 16.8; p=0.415) at month 36 when compared with sham control.
Mean NEI-VFQ-25 DSS score over time for all patients (mean±SE). Baseline DSS: quartile 1, 0–25; quartile 2, >25–50; quartile 3, >50–75; quartile 4, >75–100. DSS, driving subscale; FAc, fluocinolone acetonide; mo, months; NEI-VFQ-25, National Eye Institute Visual Functioning Questionnaire-25.
Mean change in driving subscale (DSS) score over 3 years by baseline DSS quartile (±SE). FAc, fluocinolone acetonide.
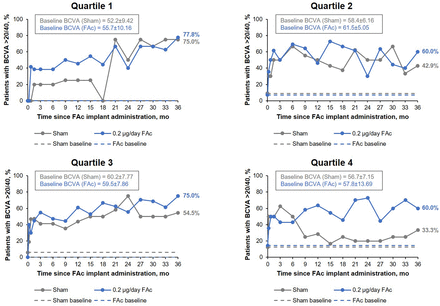
When the percentage of patients achieving BCVA of 20/40 or better was compared for the FAc-treated and sham-treated groups, no significant difference was seen throughout the study period (figure 3). At year 3, the percentage of patients achieving BCVA of 20/40 or better in quartile 1 was similar for both treatment groups; 77% (FAc) vs 75% (sham). Similar results were observed for quartile 1 for contrast sensitivity, with a mean difference of 4.4 (95% CI −4.0 to 12.8; p=0.278) between FAc-treated and sham-treated groups.
Percentage of patients achieving best-corrected visual acuity (BCVA) of 20/40 or better over time, by baseline NEI-VFQ-25 driving subscale score quartile. FAc, fluocinolone acetonide; mo, months; NEI-VFQ-25, National Eye Institute Visual functioning Questionnaire-25.
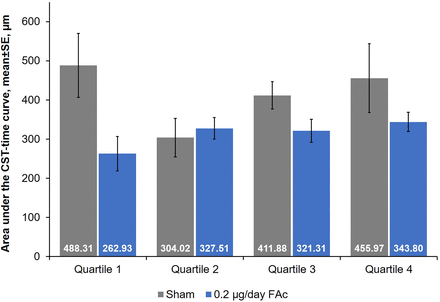
Control of oedema over time was compared between treatment groups using the AUC based on CST, where the AUC is divided by the number of days of follow-up (ie, 3 years≈1095 days). Differences in the control of oedema between FAc-treated and sham-treated patients were only significant for quartile 1 (p=0.04; figure 4).
Control of oedema over 36 months by baseline driving subscale (DSS) score quartile. AUC, area under the curve; CST, central subfield thickness; FAc, fluocinolone acetonide.
At year 3, significant improvements in the near activities, mental health, role difficulties and dependency subscales of NEI-VFQ-25 were observed for patients in quartile 1 treated with FAc when compared with the sham group (p≤0.025; table 1).
Mean (SE) change from baseline in NEI-VFQ-25 subscale scores for quartile 1 at year 3: comparison of sham control and 0.2 µg/day FAC arms
Discussion
Vision impairment associated with DMO affects both an individual’s decision to drive and perception of difficulty with driving. This, in turn, affects an individual’s QoL, independence and general well-being. Previous studies of populations of elderly patients have noted a correlation between driving cessation and poor health outcomes, particularly an increase in depressive symptoms.14 15 Therefore, treatments that improve vision have the potential to substantially improve a patient’s QoL. In the FAME trials, FAc implants provided long-term benefit to patients with DMO, significantly improving BCVA.10 However, anatomical and visual acuity measures do not describe the total treatment benefit experienced by patients or the total array of effects that improved vision can have on patients’ lives. Vision-related patient-reported outcome measures, such as NEI-VFQ-25, can capture treatment effects that are not reflected with clinical measures such as BCVA and CST.
The aim of this post hoc analysis of the FAME study was to explore a possible correlation between improvements in anatomic and visual acuity outcomes with a patient’s decision to drive and perception of driving difficulty using the NEI-VFQ-25 DSS. As vision-related patient-reported outcomes are affected by the better-seeing eye, especially when considering binocular activities such as driving,26 it was important to limit potential bias in this post hoc analysis by only using data from patients whose study eye had better or equal BCVA compared with the non-study eye at randomisation and who had not indicated that they had never driven.
In the overall population, the greatest improvements in numerical DSS scores at year 3 were observed for patients who had received an FAc implant. This observation aligns with previously published post hoc findings for the antivascular endothelial growth factor treatment, ranibizumab (Genentech, South San Francisco, California, USA), in which patients not driving at baseline were more likely to be driving following treatment than those treated with sham or laser.20 Although direct comparisons between these findings cannot be made due to differences in trial design, population sizes and dosing schedule, interventions aimed at consistently improving vision and reducing oedema can evidently have a wider impact on patients’ lives. Importantly, the analyses for ranibizumab are post hoc and based on the phase III clinical trial where a monthly dosing regimen was employed.
To adjust for potential ceiling effects in patients who have a high baseline DSS score, the population was divided into quartiles according to their baseline DSS scores. The greatest treatment response was seen in quartile 1, the group with the lowest baseline DSS scores (0–25). Surprisingly, this observation was not related to differences in achieving BCVA of 20/40, or improvements in contrast sensitivity. Significant differences were seen in the control of oedema between FAc-treated and sham-treated eyes in quartile 1, indicating a possible correlation between improvements in DSS scores at year 3 and control of oedema. These differences among the quartiles could also partially be due to the greater baseline oedema and lower vision, and therefore greater potential for improvement in oedema and vision in quartile 1. FAc-treated patients in quartile 1 also displayed significant improvements compared with sham across the following NEI-VFQ-25 subscales: near activities, mental health, role difficulties and dependency. The wider impact reported here may reflect changes in patient outlook and experience. By gaining more independence through driving or performing tasks after treatment, individuals may become less dependent on others and more confident in their abilities and vision. This could have a positive impact on their mental health and encourage their decision to return to driving.
Although this exploratory analysis of FAc links improvements in DSS score to clinical outcomes, the treatment response appears to be related to control of oedema rather than central visual function as assessed by BCVA. This supports previous findings by Freeman et al who found that the relationship between visual functions and changes in driving habits is complex and related to the type of visual function affected rather than simply reduction in visual acuity.27
FAME was not designed or powered to determine the relationship between FAc treatment effects and driving, so these findings must be viewed in the context of the limitations associated with post hoc analyses and small patient numbers, particularly in each of the four quartiles. As noted by the investigators for the pooled analysis of the RESTORE, RIDE and RISE trials,20 the DSS used here does not offer insight into how safe a driver may be or the difficulties they may encounter while driving. It is a subjective, self-reported measure that a patient uses to comment on their own driving abilities; not a measure of whether their driving skills are better than an individual who did not receive treatment. This study is dependent on patient self-reporting ability which may be limited by patient mistakes and being a poor historian. Patient self-reporting ability is also limited by the ability of the patient to accurately self-assess if they can safely drive, as patients may face obstacles not uncovered by NEI-VFQ-25 subscales.
The 0.2 µg/day FAc implant offers the potential to control DMO over multiple years with a single administration, creating a foundation on which intermittent adjunctive treatment may be added depending on the severity and course of disease. This exploratory analysis highlights the broader benefits of sustained oedema control on patients’ lives, beyond improvement and stabilisation of central visual acuity. Additional studies to evaluate the correlation between control of oedema associated with 0.2 µg/day FAc implants and effects on other QoL parameters are in progress, based on the results of this exploratory, post hoc analysis. Future work will expand on these findings and may highlight benefits beyond improvements in measured visual acuity.
Acknowledgments
The authors would like to thank the patients who participated in the FAME trials. Medical writing assistance was provided by Helios Medical Communications, Alderley Edge, Cheshire, UK and supported by Alimera Sciences. Biostatistical analyses were undertaken by Barry Kapik, formerly Alimera Sciences.
References
Footnotes
Contributors DSG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the acquisition, analysis or interpretation of data and critical revision of the manuscript for important intellectual content.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests DSG reports consultancy with Alimera Sciences and EyePoint Pharmaceuticals. SMH reports consultancy or speaker’s bureau membership with EyePoint Pharmaceuticals, Alimera Sciences, Ocular Therapeutix, Alcon, Allergan, OD-OS, Sandoz-Novartis, Spark Therapeutics, Regeneron and Clearside Biomedical. IJS reports consultancy or speaker’s bureau membership with Alimera Sciences, Allergan, Genentech, Novartis and Regeneron.
Patient consent for publication Not required.
Ethics approval The protocol was approved by the institutional review board/ethics committee at each study site, and the trials were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon request.